| Search for content and authors |
Synthesis of new 5H-indolo[2,3-b]quinoline derivatives with a high selective cytotoxic activity |
| Katarzyna Sidoryk 1, Marta Świtalska 2, Piotr Cmoch 1,3, Monika Kaczmarska 1, Iwona Bujak 1, Joanna Wietrzyk 2, Krzysztof Bańkowski 1, Łukasz S. Kaczmarek 1 |
|
1. Pharmaceutical Research Institute, Rydygiera 8, Warsaw 01-793, Poland |
| Abstract |
| 5,11-dimethyl-5H-indolo[2,3-b]quinoline (DiMIQ), the synthetic analogue of the neocryptolepine, displays high cytotoxic activity, which is comparable to the cytotoxic activity of doxorubicin, but the high toxicity of DiMIQ and its low bioavailability inspired us to look for new analogues of DiMIQ. Numerous examples in recent literature and first results of our investigations reveal that the lowered toxicity of DiMIQ can be achieved by a construction of a conjugate composed of DiMIQ and amino acids or peptides. Also the strongly hydrophilic guanidinium group can increase the bioavailability of new conjugates, and it can modulate their cytotoxic activity, and can increase their selectivity. Now, we designed and obtained a series of novel hybrid compounds composed of 5H-indolo[2,3-b]quinoline and the guanidinium group or a residue of a N-guanylamino acid. All the new conjugates displayed a high cytotoxic activity against cell lines: non-small cell lung cancer A549, breast cancer MCF-7, colon cancer LoVo, cervix carcinoma KB. The best compound (I) displays also >1000 – fold more potent cytotoxic activity against all the cancer cell lines than for normal mice fibroblasts BALB/3T3. 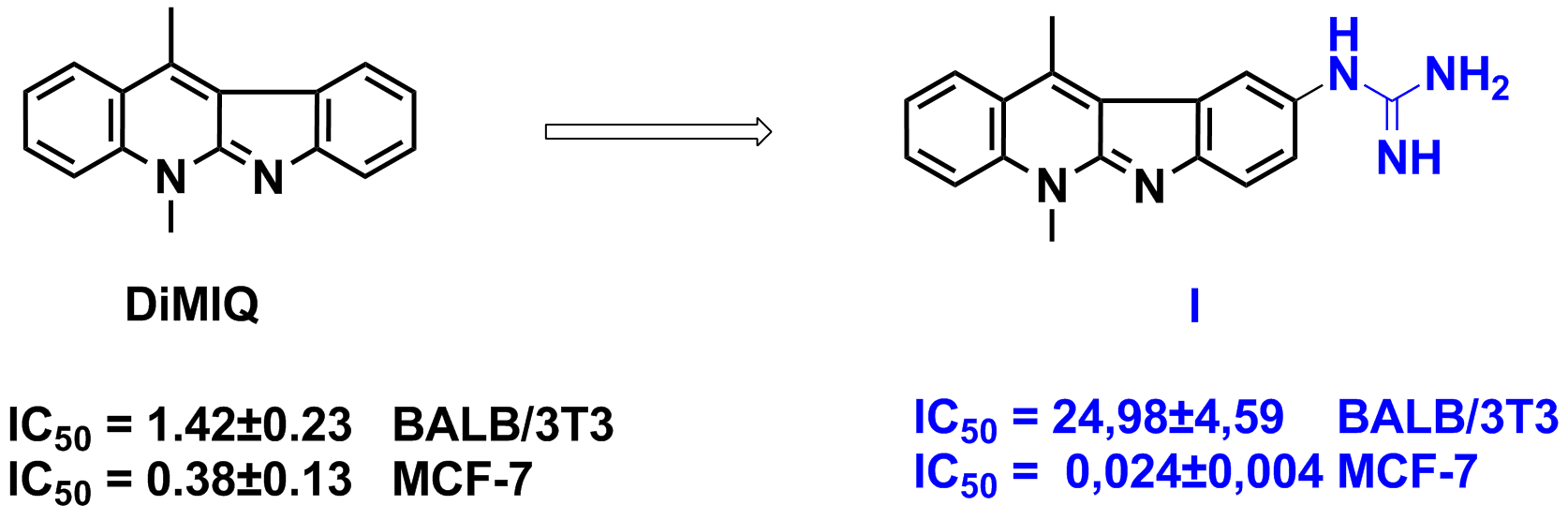
IC50 - compound concentration leading to 50% inhibition of cell proliferation [μg/mL] |
| Legal notice |
|
| Related papers |
Presentation: Poster at IX Multidyscyplinarna Konferencja Nauki o Leku, by Katarzyna SidorykSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-30 21:56 Revised: 2014-05-02 18:56 |