| Search for content and authors |
N-terminal amidinated derivatives of the cyclic 1,4-ureido-deltorphin analogues: the synthesis and receptor binding studies |
| Krzysztof Bańkowski 1, Olga M. Michalak 1, Anna A. Leśniak 2, Katarzyna E. Filip 1, Jan Izdebski 3 |
|
1. Pharmaceutical Research Institute, Rydygiera 8, Warsaw 01-793, Poland |
| Abstract |
Cyclic ureidopeptides, analogues of deltorphine tetrapeptide (Formula I), were amidinated in hope of preparing new derivatives with high resistance to enzymatic degradation, interesting opioid receptor binding profile and potentially improved blood-brain barrier permeability. 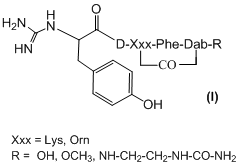
Amidination of corresponding cyclic ureidopeptides was performed using two reagents: N,N’-bis-Boc-S-methyl-isothiourea and 1H-1-pyrazole-1-carboxyamidine (with the latter generally giving better results). The new analogues were purified by reverse-phase HPLC and their purity and structural identity were established by analytical HPLC, HR-MS, and NMR studies. Binding affinities for μ and δ opioid receptors of new amidinated analogues as well as of their no-amidinated precursors were determined by competitive binding assaysin the presence of the selective radioligands: [3H]DAMGO (selective µ receptor agonist) and ([3H]Ile5,6 deltorphin II) ([3H]DELT II (selective δ agonist). Most of amidinated and no-amidinated cyclic ureidopeptides possess mixed µ agonist/δ agonist profile and have therapeutic potential as analgesic. |
| Legal notice |
|
| Related papers |
Presentation: Poster at IX Multidyscyplinarna Konferencja Nauki o Leku, by Krzysztof BańkowskiSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-13 09:44 Revised: 2014-04-27 07:10 |