| Search for content and authors |
PRIMARY CYTOTOXICITY EVALUATION OF NEW HETEROCYCLIC SYSTEMS WITH PYRIDODIAZEPINE. |
| Wanda Nawrocka 1, Hanna Liszkiewicz 1, Andrzej Dryś 2, Joanna Wietrzyk 3, Adam Opolski 3 |
|
1. Akademia Medyczna, Katedra Technologii Leków, Nankiera 1, Wrocław 50-140, Poland |
| Abstract |
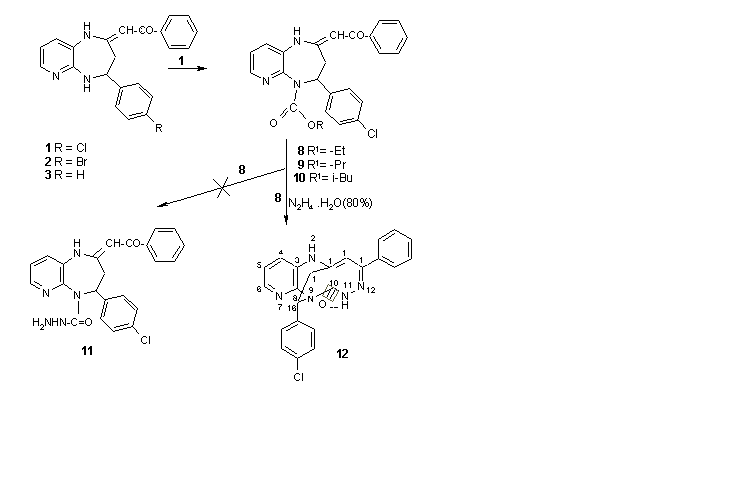
New esters 8-10 and hydrazones 4-6 were synthesized from 1-phenyl-2-(4-aryl-1,3,4,5-tetrahydropyrido[2,3-b][1,4]diazepin-2-ylidene)ethanones (1-3). Subsequent treatment of hydrazone 4 with p-chlorobenzaldehyde furnished azine 7. The prolonged heating of ester 8 with hydrazine hydrate afforded a polyheterocyclic compound with fused and bridged rings 12. The structures of new compounds were determined by analytical and spectral (IR, 1H NMR, MS) methods. Compounds 8 - 10 and 12 were examined for their antiproliferative activity in vitro against the cells of 5 human cancer cell lines, using SRB or MTT technique. One out of all tested compounds 12 revealed cytotoxic activity in vitro against all cell lines applied with ID50 (inhibitory dose 50%) values lower than 4 mg/ml, which is an international activity criterion for synthetic compounds. All compounds inhibits the proliferation of HL-60 human promyelocytic leukemia cell line . Carbamates 9 and 10 were active only against two cancer cell lines, namely HL-60 leukemia and HCV-29T urinary bladder cancer. Compound 8 revealed activity only against HL-60 leukemia cells.
 |
| Legal notice |
|
| Related papers |
Presentation: Poster at V Multidyscyplinarna Konferencja Nauki o Leku, by Wanda NawrockaSee On-line Journal of V Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2006-04-11 07:53 Revised: 2009-06-07 00:44 |