| Search for content and authors |
1D ZnO-based structures obtained by thermal oxidation of ZnTe and ZnTe/Zn nanowires |
| Katarzyna Gas 1, Eliana Kamińska 2, Elzbieta Dynowska 1, Sławomir Kret 1, Maciej Wiater 1, Wojciech Zaleszczyk 1, Agnieszka Kamińska 3, Tomasz Wojtowicz 1, Wojciech Szuszkiewicz 1 |
|
1. Polish Academy of Sciences, Institute of Physics, Lotnikow 32/46, Warsaw 02-668, Poland |
| Abstract |
| One-dimensional (1D) semiconductor nanostructures like, e.g., nanowires (NWs) or nanobelts recently attracted a great deal of attention due to their potential application in electronic and optoelectronic nanodevices. Among many materials zinc oxide is particularly intensively researched due to its wide direct-gap (3.37 eV at 295 K) and a large exciton binding energy of 60 meV. The non-intentionally doped ZnO usually exhibits n-type conductivity and it is difficult to convert it to p-type semiconductor. The inherent difficulties in obtaining p-type ZnO is the major obstacle in widespread device applications of this material. Contrary to ZnO, ZnTe can be relatively easy highly p-type doped. Moreover, as it was already shown thermal oxidation of nitrogen-doped ZnTe allows to obtain p-type ZnO [1, 2], therefore one possible way to produce p-type, ZnO-based NW structure would be through the oxidation of p-type ZnTe NWs. In this paper we report on fabrication and characterization of 1D ZnO-based structures. The nanostructures have been obtained by thermal oxidation of ZnTe and ZnTe/Zn core/shell NWs. The ZnTe NWs were grown on GaAs substrates by molecular beam epitaxy employing a gold-catalyzed vapor-liquid-solid mechanism [3, 4]. After the growth, the ZnTe-based NWs are annealed under different conditions: we vary the annealing time, temperature and atmospheres (O2, Ar or mixture O2 and Ar). The surface morphology of the nanostructures before and after thermal annealing process is examined by scanning electron microscopy. The structural characterization is performed by using transmission electron microscopy and X-ray diffraction. The energy dispersive X-ray spectroscopy is applied to examine the chemical composition of the resulting nanostrucrures. The optical characterization is carried out by room temperature micro-Raman spectroscopy, as well as, by cathodoluminescence. The annealing of ZnTe NWs at 300°C for 4 h in O2 led to formation of numerous 30-60 nm in diameter ZnO nanocrystals on the surfaces of the wires, whereas the same annealing procedure applied to ZnTe/Zn core/shell NWs does not change their morphology significantly (see Figure 1), indicating that the ZnTe/ZnO core/shell NWs are obtained in this case. The two-step oxidation process for core/shell ZnTe/Zn NWs in O2 (in the first step the samples are annealed at 350°C by 1h and after that the temperature is increased to 400°C and oxidation process is continued by 1h) results in formation of nanotubes composed mostly of small ZnO and also of TeO2 crystallites with random crystal orientations. These randomly oriented ZnO nanocrystals can get oriented along the crystal orientation of the Zn shell initially covering the ZnTe/Zn NWs when after normal oxidation they are subjected to 600°C argon annealing. Moreover, an additional annealing at 600°C in argon causes branching on the NWs into only a several nm in diameter but almost a micrometer long dendrites-like very straight and uniform nanowires. Presence of the gold droplets at the end of each wire indicates the same Au-driven catalytical grows mode. The annealing of the ZnTe NWs in O2 at the temperature above 500°C destroys completely the NWs. However, we can increase the oxidation temperature up to 600°C by changing the annealing atmosphere to the mixture of 2% O2 and 98% Ar. Thanks to such approach the obtained NWs contain only ZnO and shows characteristic for ZnO near-band edge and defect luminescence. 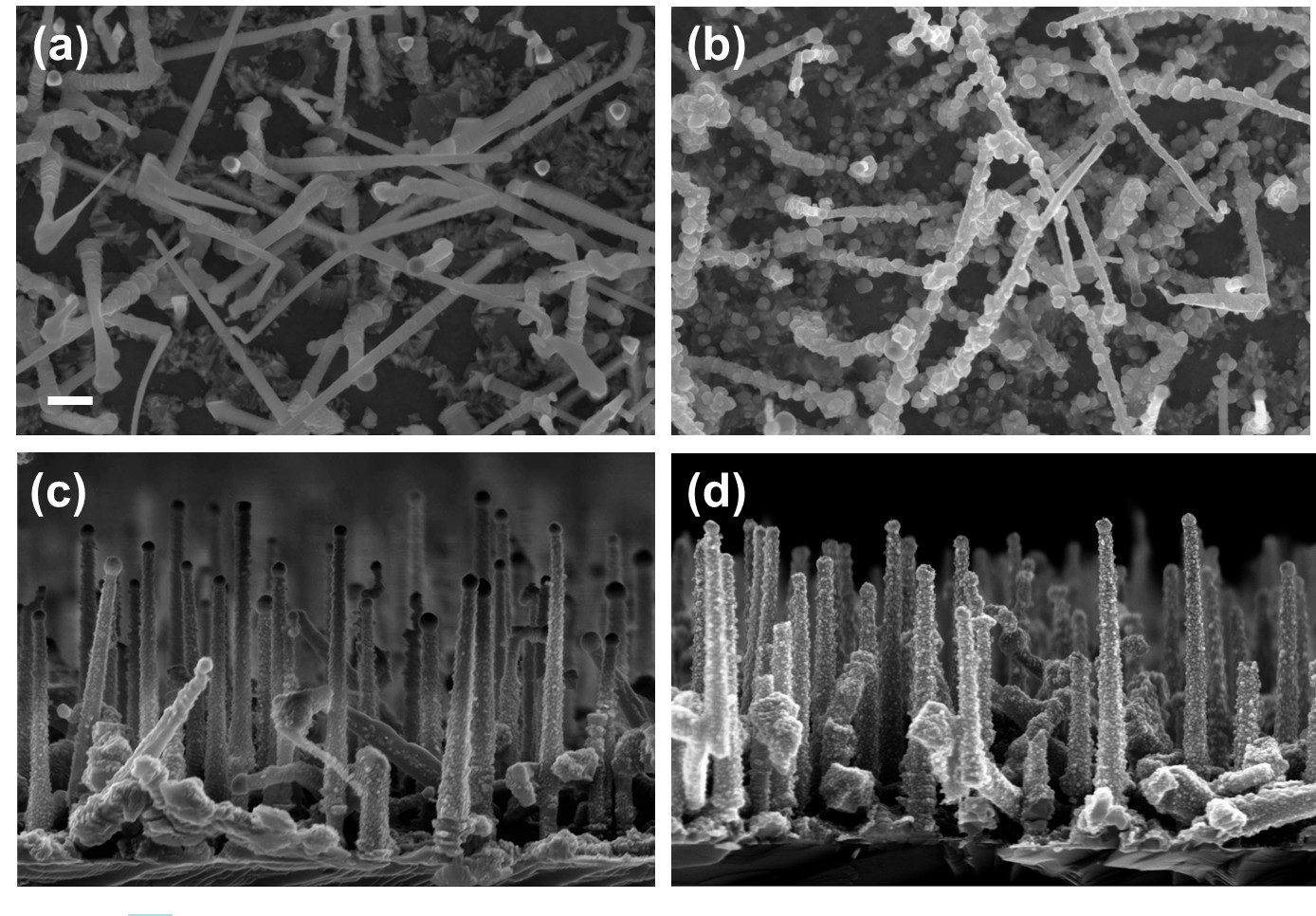 Figure 1: SEM images of (a) ZnTe NWs (b) oxidized ZnTe NWs (c) ZnTe/Zn core/shell NWs (d) oxidized ZnTe/Zn core/shell NWs. The NWs are oxidized in O2 at 300°C for 4 h. The scale bar on the SEM picture corresponds to 200 nm. The studies were partially supported by the European Union within European Regional Development Fund, through grant Innovative Economy (POIG.01.01.02-00-008/08). |
| Legal notice |
|
| Related papers |
Presentation: Poster at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, General Session 8, by Katarzyna GasSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-04-15 22:58 Revised: 2013-07-17 13:36 |