| Search for content and authors |
Analytical control of synthesis and determination of BR-S by HPLC |
| Joanna Zagrodzka , Łukasz S. Kaczmarek , Ewa Kyrcz , Justyna Kowalska , Katarzyna Badowska-Rosłonek , Łukasz Mucha |
|
Pharmaceutical Research Institute (IF), Rydygiera 8, Warszawa 01-793, Poland |
| Abstract |
BR-S namely (5R)-5-ethylamino-3-(3-methoxypropyl)-2,2-dioxo- 2,6,9-dithia-3-azabicyclo [4.3.0]nona-7,10-diene-8-sulfonamide is a carbonic anhydrase inhibitor used to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension. 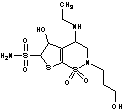
BR-S can be obtained by the seven-step synthesis. Analytical method used for this purpose must ensure fast and efficient determination of the presence of starting materials, product, impurities. The common and widely applied method which meets these requirements is high performance liquid chromatography, especially in reverse phase mode (RP-HPLC). This technique was used for optimization of synthesis and determination of purity of the API. Finally an HPLC method was elaborated. In course of extensive research it was proven, that the developed method is selective and sensitive for all separated compounds. It fulfills the criteria of European Pharmacopoeia for API analysis and can be used to control the synthesis process. |
| Legal notice |
|
| Related papers |
Presentation: Poster at VII Multidyscyplinarna Konferencja Nauki o Leku, by Joanna ZagrodzkaSee On-line Journal of VII Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2010-03-18 17:27 Revised: 2010-05-04 20:16 |