| Search for content and authors |
Separation of enantiomers of Z and E 3-phenylglycidic acid t-butyl ester |
| Magdalena Jezierska-Zięba 1, Barbara Kąkol 1, Michał Fedoryński 2, Jacek Cybulski 1 |
|
1. Industrial Chemistry Research Institute (ICRI), Rydygiera 8, Warszawa 01-793, Poland |
| Abstract |
Looking for a new method of synthesis of (2R,3S)-3-phenylisoserine hydrochloride – an important intermediate on the route to Paclitaxel – we turned our attention to the reaction of (2R,3R)-3-phenylglycidic acid esters with ammonia, which should lead to (2R,3S)-3-phenylisoserine.
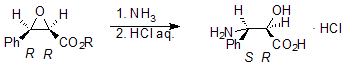 Glycidic esters are most conveniently prepared in Darzens reaction of α-halogenoacids esters with carbonyl compounds, carried out in the presence of a base. Working on a practical synthesis of (2R,3R)-3-phenylglycidic acid t-butyl ester we needed a good analytical method of determination of the content of enantiomers of both Z and E isomers of 3-phenylglycidate in reaction mixtures. The aim of the presented work was to elaborate the conditions for chiral separation of the enantiomers mentioned using high performance liquid chromatography (HPLC). We tested three analytical columns and investigated the chiral separation depending on the composition of the mobile phase. The following mixtures were used as mobile phases in different proportions: n-hexane / 2-propanol, n-hexane / methanol, n-hexane / t-butyl methyl ether. The best chiral separation of the enantiomers of Z and E 3-phenylglycidic acid t-butyl ester was achieved using the following conditions: |
| Legal notice |
|
| Related papers |
Presentation: Poster at VI Multidyscyplinarna Konferencja Nauki o Leku, by Magdalena Jezierska-ZiębaSee On-line Journal of VI Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2008-03-14 08:36 Revised: 2009-06-07 00:48 |