| Search for content and authors |
Hexenoses in design of glycoconjugates – from chemistry to function |
| Piotr Krzeczyński 1, Grzegorz Grynkiewicz 1, Wiesław Szeja 2 |
|
1. Pharmaceutical Research Institute (IF), Rydygiera 8, Warszawa 01-793, Poland |
| Abstract |
Isoflavones have received a great deal of attention over the last decades as possible drug leads with demonstrated activity against multiple targets. However, their poor bioavailability and quick metabolism result in low efficacy and call for well designed synthetic modifications before potential medicinal application. Our lasting interest in unsaturated pyranosides prompted syntheses of various isoflavone glycosides, exemplified by genistein derivative IF021, containing hex–2–enopyranose moiety. 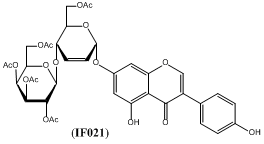
Although IF021 was designed as genistein pro-drug, and similar effects in cell tests were predicted, it turned out that it has distinctly different biological activity profile than the parent isoflavone [1; 2] with unexpected cytotoxicity related to interference with the mitotic spindle dynamics. Despite our efforts, laboratory synthesis of IF021 proved poorly stereoselective, offering no hope for elaboration of efficient manufacturing process. Considering hex-2-enopyranosides as the new type of saccharide scaffold which may be useful in medicinal chemistry, we have decided to examine synthetic methods of its linkage to selected polyphenolic substrates by several strategies involving Ferrier rearrangement and transition metals promoted glycosylations. Apart from straight isoflavone – glycon bonding, variety of linkers were tried for obtaining O-linked or C-linked glycoconjugates. Generally, all types of unsaturated isoflavone glycoconjugates exhibited specific influence on cell cycle, which warrants more specific pharmacological research.References: Acknowledgement: |
| Legal notice |
|
| Related papers |
Presentation: Oral at IX Multidyscyplinarna Konferencja Nauki o Leku, by Piotr KrzeczyńskiSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-18 12:03 Revised: 2014-05-02 12:25 |