| Search for content and authors |
Simultaneous CE determination of counterion and possible impurity from synthetic route in pharmaceutical substance prasugrel hydrochloride. |
| Wioleta Maruszak , Marcin Cybulski |
|
Pharmaceutical Research Institute, Rydygiera 8, Warsaw 01-793, Poland |
| Abstract |
The determination of small organic and inorganic ions is an important part of pharmaceutical analysis. Many drug molecules are charge and are manufactured with counterion. Hence, it is important to analytically characterize the drug stoichiometry to ensure that the potency of the batch of drug substance is known. On the other hand small organic and inorganic ions could be a contaminant impurities coming from the synthetic route and the task of impurity determination in drug is of principle importance. Prasugrel is the most recent member of the thienopyridine class of antiplatelet agents1. Similarly to other thienopyridines, it is commonly used in a therapy of choice for patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) with stent implantation2,3. To date, the registered medication brands with prasugrel contain the active substance (API) in the form of hydrochloride salt. The last steps in our synthetic procedure comprise the O-acetylation of 5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one (3) to the compound 4, in basic reaction conditions and a presence of acetic anhydride, then the formation of prasugrel salt. During coupling reaction workup the acetic anhydride may convert to acetic acid or its salts followed the acetic acid formation as an impurity in final procedure for prasugrel hydrochloride obtaining. 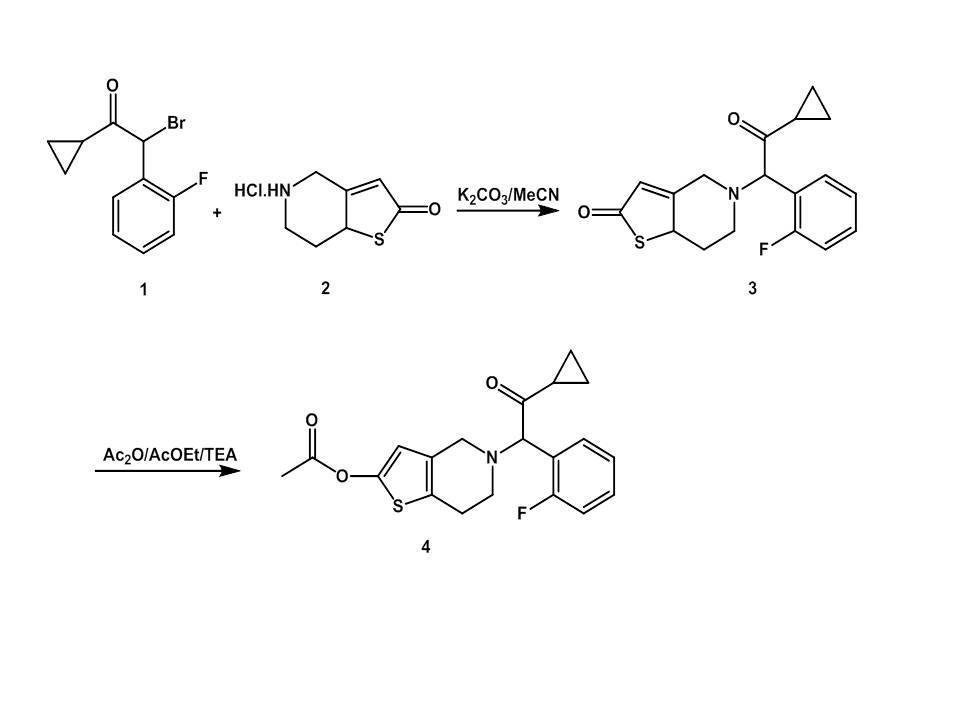
The determination of small organic and inorganic ions in pharmaceutical analysis include mainly ion chromatography (IC), flame atomic absorption spectrometry and flask-based methods e.g. titration which are expensive, time, reagent and cost consuming. Because such ions usually have little or no chromophore can be analyzed by indirect UV detection. This detection mode could be performed using capillary electrophoresis technique (CE). During the past decade many original and review papers as well as monographs mention about the couterions or impurities determination in drugs. But there was not found the work described validated method for simultaneous conterion and impurity ion determination in pharmaceutical substance. This work present the potential advantages and validation of CE method for the simultaneous determination of counterion and possible impurity from synthetic route in prasugrel hydrochloride. Acknowledgements: Research project has been supported by European Union (under European Regional Development Fund) UDA-POiG.01.03.01-14-062/09-00 “Innovative technologies of cardiovascular medicines of special therapeutic and social importance” 
References: |
| Legal notice |
|
| Related papers |
Presentation: Poster at IX Multidyscyplinarna Konferencja Nauki o Leku, by Wioleta MaruszakSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-13 11:01 Revised: 2014-05-02 12:53 |