Construction of molecules exhibiting controlled directional motions at the molecular level is, at present, a great scientific challenge. Such motions can be realised in several model molecules, for example, bistable catenanes and rotaxane. In order to control molecular devices one has to understand factors influencing the motions and the nature of interactions defining the mechanical bonds in model systems.
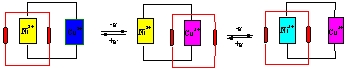
We have recently proposed a new type of catenanes consisting of bismacrocyclic transition metal complexes linked by aliphatic chains and interlocked with substituted crown ether. Under an external stimuli - electrochemical pulses - they exhibit controlled intramolecular relocation of the crown ether between two positions. This relocation is possible due to π...π interactions between the aromatic fragments of the crown ether and the metal centres (Ni, Cu) embedded in the macrocyclic rings [1-4].
By changing properties of components of mechanically bonded molecules, one can modify the properties of resulting complex structures. Such a tuning of properties can be achieved by severe measures as, for example, replacement of the metal ions, elongation of the aliphatic linkers or change of macrocycles. More subtle changes of properties can be introduced by proper chemical modifications of particular positions of the parent systems.
The development of appropriate physical methods useful for monitoring the dynamics of supramolecular systems is currently of utmost importance. We demonstrate using voltammetry that interlocking of the dibenzocrown ether with the homodinuclear bismacrocyclic transition metal complexes leads to increased stability of the mixed-valence states, which was reflected in higher values of comproportionation constants We present a heterodinuclear bismacrocyclic transition metal complex exhibiting potential-driven intramolecular motion of the interlocked crown ether unit. We would like to point out here the unique ability of the Osteryoungs square wave and reverse pulse techniques to detect potential triggered intramolecular motion. By applying appropriate potentials either copper or nickel (or both) are reversibly oxidized to the higher (+3) oxidation state. This favours interaction with the π-electron-rich aromatic system of the crown unit which relocates the crown towards the oxidized metal center.
[1] B. Korybut-Daszkiewicz, A. Więckowska, R. Bilewicz, S. Domagała, K. Woźniak, J. Am. Chem. Soc. 2001, 123, 9356.
[2] A. Wieckowska, R. Bilewicz, S. Domagala, K. Wozniak, B. Korybut-Daszkiewicz, A. Tomkiewicz, J. Mrozinski, Inorg. Chem. 2003, 429, 5513.
[3] B. Korybut-Daszkiewicz, A. Więckowska, R. Bilewicz, S. Domagała, K. Woźniak, Angew. Chem., Int. Ed. Engl. 2004, 116, 1700.
[4] S. Domagała, A. Więckowska, J. Kowalski, A. Rogowska, J. Szydłowska, B. Korybut-Daszkiewicz, R. Bilewicz, K. Woźniak, Chemistry Eur. J. 2006, 12, 2967.
|