| Search for content and authors |
Polymorphism control of pharmaceutical compound acetaminophen by ultrasonic irradiation |
| Yoichiro Mori 1, Yoshinori Takahashi 1,2, Kenji Ikeda 1, Toshihiko Yamada 1, Ken Nishimura 1, Mihoko Maruyama 1, Hiroshi Yoshikawa 1,3, Shino Okada 2, Hiroaki Adachi 1,2, Shigeru Sugiyama 4, Hiroyoshi Matsumura 1,2, Kazuhumi Takano 2,5, Tsuyoshi Inoue 1,2, Satoshi Murakami 2,6, Masashi Yoshimura 1, Yusuke Mori 1,2 |
|
1. Graduated School of Engineering, Osaka University (OSAKAUNIV), Osaka, Japan |
| Abstract |
Polymorphs are solid phases in which the chemical composition is equal but the crystal structure differs. In the pharmaceutical industry, especially, most of compounds have polymorphs, and polymorphism control is very important because different polymorphs exhibit different stabilities, drug effects, formation properties, etc. Acetaminophen (Figure 1), which is one of the typical pharmaceutical compounds, has three known polymorphs, numbered I, II and III. The stable form I is the commercially used form because of its stability. In contrast, the metastable form II has been shown to be more soluble and more readily compressible into tablets than form I, and controlled crystallization of the form II is an important issue for practical use. Hence we attempted to control polymorphism of acetaminophen form II by ultrasonic irradiation in this study. Acetaminophen aqueous solutions (32 mg/ml, total 50 bottles) were prepared, cooled to 0°C at a constant cooling rate (3°C/h), maintained at that temperature for about 2 days, and irradiated with ultrasound with different frequencies (no irradiation, 28, 45, 100 kHz) for investigating the effect of crystallization and polymorphism control. Figure 2 shows the probability of nucleation and form II crystallization on each condition. In the cases of no irradiation and 100 kHz irradiation, nucleation did not occur in solutions (no irradiation: 0/20 (0%), 100 kHz: 0/10 (0%)). In contrast, ultrasonic irradiation with the frequency of 28 or 45 kHz promoted the nucleation (28 kHz: 9/9 (100%), 45 kHz: 10/10 (100%)), and also increased probability of the form II crystallization as shown in Figure 2 (28 kHz: 6/9 (67%), 45 kHz: 6/10 (60%)). 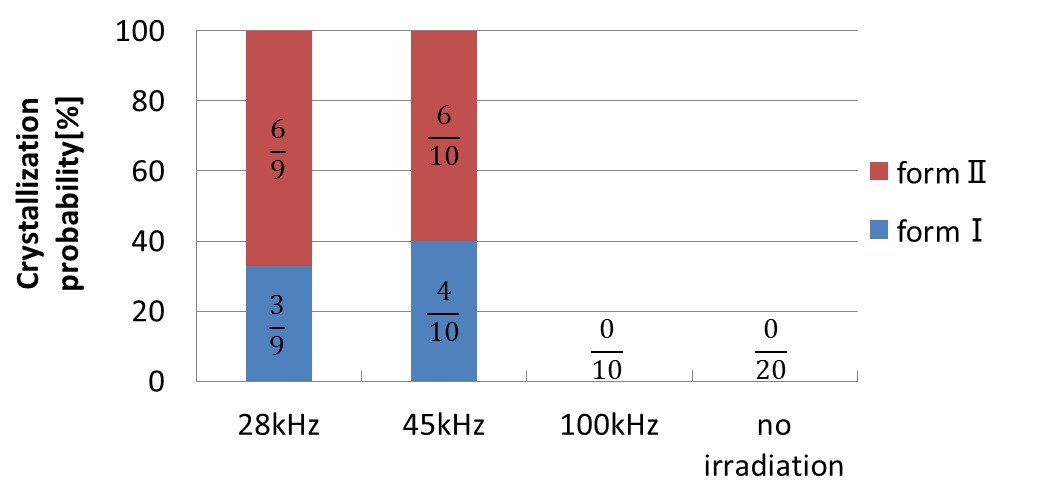
Fig. 2. Frequency dependence of crystallization probability |
| Legal notice |
|
| Related papers |
Presentation: Oral at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, General Session 3, by Yoichiro MoriSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-04-15 18:42 Revised: 2013-07-16 17:03 |