| Search for content and authors |
The optimization of solution flow rate producing high-quality and large protein crystals |
| Yuki Hayashi 1, Mihoko Maruyama 1, Satoshi Nakayama 1, Yoshinori Takahashi 1, Hiroshi Yoshikawa 2, Masashi Yoshimura 1, Masaru Tachibana 3, Haruhiko Koizumi 4, Sigeru Sugiyama 5, Hiroaki Adachi 1,6, Kazufumi Takano 6,7, Satoshi Murakami 6,8, Hiroyoshi Matsumura 1,6, Tsuyoshi Inoue 1,6, Yusuke Mori 1,6 |
|
1. Graduated School of Engineering, Osaka University (OSAKAUNIV), Osaka, Japan |
| Abstract |
Large protein crystals with high-quality are essential for determining the three-dimensional structure of proteins by X-ray diffraction measurement and neutron diffraction measurement. We have developed the top-seeded solution growth with the floating and solution-stirring technique (TSSG-FAST)1 and succeeded to grow crystals of HIV-1 protease suitable for neutron diffraction measurement (Fig. 1). Using these crystals, structual information about the H atoms of HIV-1 protease was successfully determined. TSSG-FAST is an efficient technique for producing high-quality and large protein crystals. To make the technique highly-developed, we studied the correlation of the growth rate and the quality with the solution flow rate, and decided the best flow rate for growing high-quality and large crystals of proteins.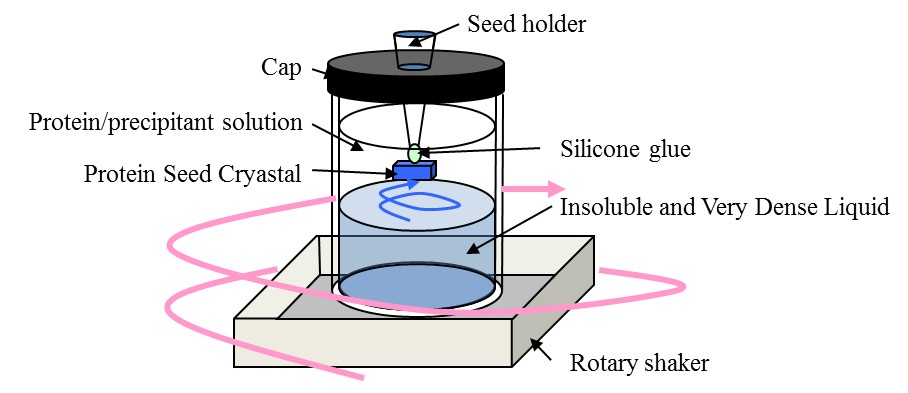 Fig. 1 Schematic illustration of the crystallization setup for TSSG-FAST Fig. 1 Schematic illustration of the crystallization setup for TSSG-FAST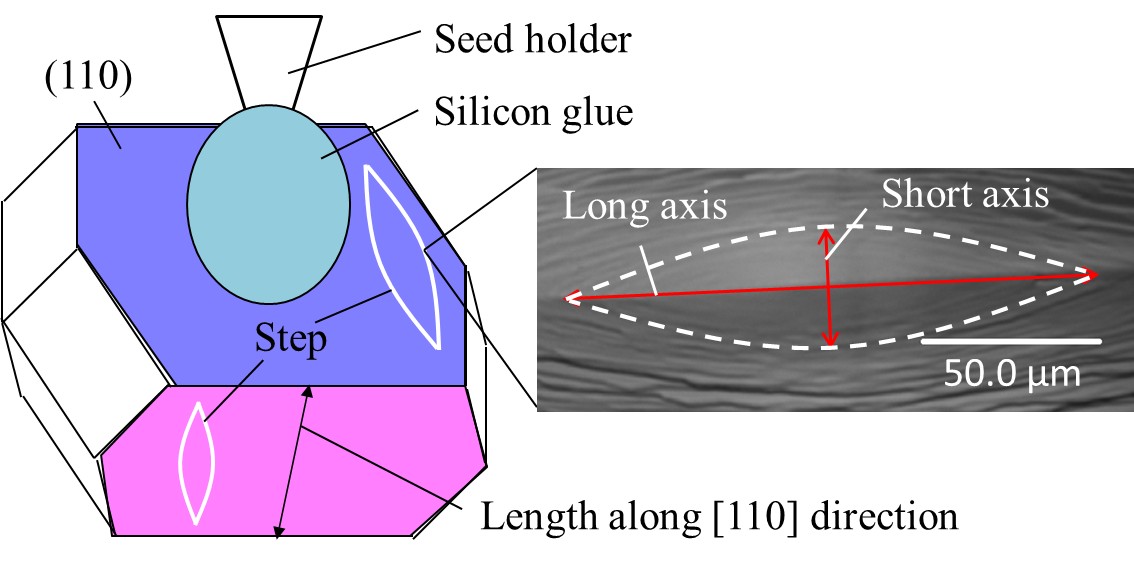 Fig. 2 Schematic illustration of the HEWL and the morphology of spiral growth hillock Fig. 2 Schematic illustration of the HEWL and the morphology of spiral growth hillock Crystals of hen egg-white lysozyme (HEWL) were grown by the TSSG-FAST under various solution flow rates, which were determined by flow analysis. Growth rate of (110) face was obtained by observing the lengths along [110] direction at the starting and the ending day (Fig. 2). We also observed the morphology of spiral growth hillocks on {110} faces by laser confocal microscopy combined with differential interference contrast microscopy (LCM-DIM). The morphology is affected by impurities and the aspect ratio (long to short axes) of steps can be used as a measure of impurity effects (Fig. 2).2 At the flow rate of 0 μm/sec, growth rate of (110) face was 0.2 μm/h and the aspect ratio of long to short axes was 4.1 ± 0.3 (Fig. 3). With increasing the flow rate, the both increased and had the maximum at the flow rate of 140 μm/sec, and then decreased. This result showed that there exists the best solution flow range, and solution flow faster than 140 μm/sec is not suitable for protein crystal growth. We consider that this result can be explained by the competition between the mass transfer of solutes and the inhibition effect of the impurities.3 
Fig. 4 Synchrotron monochromatic-beam X-ray topography (a)without stirring (b)TSSG-FAST Reference |
| Legal notice |
|
| Related papers |
Presentation: Oral at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, General Session 3, by Yuki HayashiSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-04-05 07:04 Revised: 2013-07-16 16:59 |