1. INTRODUCTION
Smelting copper and other metals actually produces arsenic as a by-product [1]. Currently, these arsenic has been simply stored because of no demand, and thus the technology required to make arsenic insoluble for safe storage has been developed vigorously. In these researches, scorodite crystals (FeAsO4∙2H2O) have been receiving attention because of their low solubility [3], and much efforts to synthesize easily scorodite are being widely performed [4-7]. Recently, novel scorodite synthesis technology by injecting O2 gas under atmospheric pressure has been reported and is attracting much attention as simple and easy synthesis method [8-11]. In this method, Fe2+ ion in solution is oxidized to Fe3+ by O2 gas injection, and scorodite crystals precipitate from the solution containing Fe3+ and As(V) ions. It has also been revealed that synthesized scorodite by this method has actually low solubility [11].
Products in this method, however, include small sized crystals with up to several μm in diameter to some extent [8]. Such small crystals dissolve more easily than large ones because of large surface area in unit volume, and the concentration of arsenic may suddenly increases in current water such as the subsurface water. In addition, such small crystals are actually grown under high super-saturation, and it is also reported that amorphous precipitations with scorodite composition having high solubility [3] are synthesized under high super-saturation [6]. Therefore, the development of new method to grow crystals with larger diameter has been required for more safety immobilization of arsenic.
Crystals with similar large particles can be synthesized under low super-saturation [12], whereas in O2 gas injection method, it is difficult to form such crystals because the supersaturation of scorodite crystal growth with the flow quantity of O2 gas is large [13].
Photocatalyst irradiated with UV light was frequently used in order to oxidize Fe2+ ions in solution [14, 15], and it also was considered that photocatalyst can easily control the oxidation rate of Fe ions, depending on the strength of UV light, and keep the super-saturation low.
Therefore, in this paper, growth of scorodite crystals has been investigated using a photocatalyst irradiated with UV light, and growth behavior of scorodite crystal in this method compared with scorodite synthesized by O2 gas injection associated with particle size.
2. EXPERIMENTAL PROCEDURE
The experiment flowchart is shown in Fig.1. Figure 2 also depicts a schematic drawing of the experimental apparatus. As2O3 derived from the copper smelting process was used as starting material. Starting material (5.28g or 2.74g) was dissolved in 40ml of deionized water and then oxidized by adding molar equivalent hydrogen peroxide to produce As (V) solution. To this arsenic solution, FeSO4∙7H2O (11.1g)(Analytical- reagent grade) and anatase TiO2 (0.500g)(Titan Kogyo Ltd. , Japan) as a photocatalyst were added. Ultraviolet light from a UV lamp (λ=365nm, 810μW/cm3 ) irradiated this solution while it was stirred at 90oC to synthesize scorodite. The solution was filtered after experiment period and a powdered product was obtained through natural drying. For comparison, scorodite crystals were also synthesized by O2 gas injection using a glass tube (inner diameter 5mm) that was just the same as the one used for synthesis by irradiation with UV light. All experiments were conducted in a glove box to ensure safety.
These products were identified by X-ray powder diffraction (XRD). Crystal morphology was observed on optical microscope and scanning electron microscope (SEM). To clarify the growth behavior, the particle density in the initial stage of reaction and the crystal size distribution (CSD) was measured. the crystal density was obtained by sampling 10μl of slurry using a microsyringe, spreading it on a glass plate to a diameter of 14mm, and then counting the number of crystals in that area. The CSD was obtained measured the ferret diameters [16] of 300 particles from the SEM photo of the filtered product.
3. RESULTS AND DISCUSSION
3.1 Product evaluation
XRD results of two products were shown in Fig.3. This figure indicates that only scorodite without another phase is synthesized by two methods. In particular, it was revealed for the first time that scorodite can grow using a photocatalyst.
Figure 4 shows SEM photo-images of synthesized scorodite. Synthesis by UV light irradiation yielded crystals with similar particle sizes. In contrast, crystals synthesized by O2 gas injection were the mixture of large and small crystals with greatly different particle diameters. We were not able to observe the difference in morphology between crystals synthesized by each of the two methods. Also, in this study, it is clearly revealed that TiO2 acted as catalyst to form the nucleation of scorodite crystal. However, we could not obtain new information on relationship between TiO2 and scorodite as well as heterogeneous nucleation of scorodite on surface of TiO2 catalysis has not been clear. Further information to clear such a relationship will be done.
3.2 Growth mechanism of scorodite crystal by photocatalyst
The color of the solution changed from pale-white to green within one hour in O2 gas injection, whereas the change in color in UV irradiation took place at 12 hours. This change depends on the amount of scorodite crystals. Therefore, this result indicates that the growth rate of scorodite by UV light irradiation is clearly slower than that of synthesis by O2 gas injection.
Measurement results of the number of crystals per unit area in the initial stage in two growth methods is shown in Table I. Although it was impossible to count the number of crystals in one hour of the reaction time by UV light irradiation because almost all of the unreacted material and TiO2 remained, in 3hours, the number of crystals by UV light irradiation was one-fifth that of O2 gas injection. In addition, it was one-fourth the numbers of crystals after one hour of reaction by O2 gas injection. This crystal density can be considered as the nucleation density in the solution.
From these experimental results, it was concluded that super-saturation is clearly lower in synthesis by UV light irradiation than in synthesis by O2 gas injection.
Figure 5 illustrates CSD curves with time for each synthesis method. Curves in UV light irradiation show single peaks and the average was 5 to 10μm, whereas CSD curves with O2 gas injection have two peaks, indicating a broader distribution of crystal sizes.
The CSD curve for UV light irradiation changed with reaction time from asymptotic to lognormal between three to five hours, but such changes occurred within three hours in O2 gas injection. An asymptotic CSD curve is expected when both nucleation and crystal growth occur, while a lognormal curve is expected when crystal growth is predominant over nucleation [17]. It is considered that the CSD curve is asymptotic at high super-saturation and changes to lognormal curve as super-saturation decreases. The CSD curve produced by O2 gas injection is asymptotic. But the CSD curve changes to lognormal curve in a short time. This reason is due to the rapid decrease of super-saturation, originated from large nucleation density and crystal growth of each nucleus crystal at high super-saturation. On the other hands, low nucleation density and slow crystal growth rate can be expected in UV light irradiation because of low super-saturation compared with O2 gas injection, and the super-saturation will decrease more slowly compared with that in O2 gas injection. As a result, the time for the CSD curve to change from asymptotic to lognormal with UV light irradiation is longer than with O2 gas injection.
As depicted in Fig. 5 (b), bimodal peaks in O2 gas injection was not observed in UV light irradiation. As for bimodal peaks, it has been reported that secondary nucleation, occurred by rapid nucleation at high super-saturation, causes such bimodal peaks in CSD [18]. Conversely, such peaks in CSD curve in UV light irradiation are not observed because of low super-saturation.
Compared with O2 gas injection, it was revealed in UV light irradiation that number of crystals with less than 5 μm in diameter is smaller, but growth rate is slower. In order to synthesize rapidly scorodite crystals with similar diameter for a short time, it is also considered that synthesis of combining UV light irradiation and O2 gas injection is required. Scorodite crystals are formed first by UV light irradiation to decrease concentration at which spontaneous nucleation can not take place, and then are grew further by O2 gas injection.
4. CONCLUSIONS
Scorodite crystals have been successfully grown using a photocatalyst irradiated by UV light for the first time. It was also revealed that these crystals in UV light irradiation were grown in lower super-saturation compared with O2 gas injection. 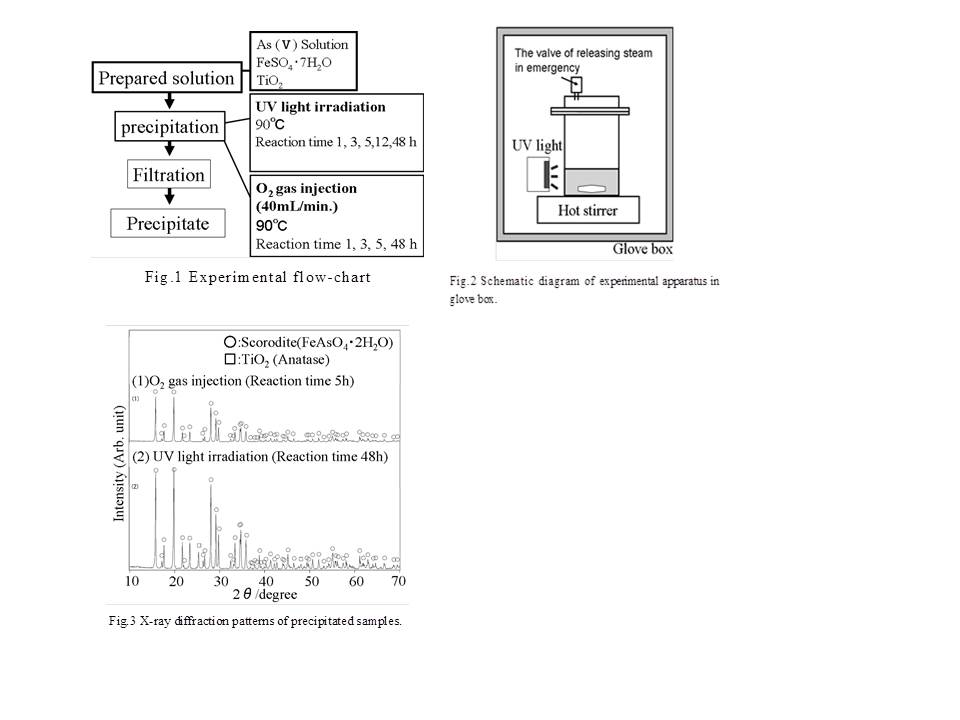

References
[1] J. E. Hoffmann, JOM, 45, 30-31 (1993).
[2] A. J. Monhemius and P. M. Swash, JOM, 51, 30-33 (1999).
[3] E. Krause and V.A. Ettel, Hydrometallurgy, 22, 311-337 (1989).
[4] M.A. Gomez, L. Becze, J. N. Cutler, and G.P. Demopoulos, Hydrometallurgy, 107, 74-90 (2011).
[5] M.A. Gomez, L. Becze, M. Celikin, and G.P. Demopoulos, J. Colloid Interface Sci., 360, 508-518 (2011).
[6] G.P. Demopoulos, D.J. Droppert, and G. Van Weert, Hydrometallurgy, 38, 245-261 (1995).
[7] D. Filippou and G. P. Demopoulos, JOM, 49, 52-55 (1997).
[8] T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Hydrometallurgy, 90, 92–102 (2008).
[9] T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Hydrometallurgy, 90, 85-91 (2008).
[10] T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Hydrometallurgy, 93, 30-38 (2008).
[11] T. Fujita, R. Taguchi, H. Kubo, E. Shibata, and T. Nakamura, Materials Transactions, 50, 321-331 (2009).
[12] H. A. Mohameed, B. Abu-Jdayil, M. Al Khateeb, Chemical Engineering and Processing, 41, 297–302 (2002).
[13] H. Okamura, bachelor thesis, Yamaguchi University (2009).
[14] W. Z. Tang and H. An, Chemosphere, 31, 4157-4170 (1995).
[15] H. Fei, W. Leng, X. Li, X. Cheng, Y. Xu, J. Zhang, and C. Cao, Environmental Science & Technology, 45, 4532–4539 (2011).
[16] T. Allen, “Particle size measurement”, Chapman and Hall, London, (1968) pp.46-48.
[17] K. Sangwal, “Additives and Crystallization Processes: From Fundamentals to Applications”, John Wiley & Sons, Inc., New York City, (2007) pp.291-295.
[18]R. Misumi, M. Tsukada, K. Nishi, and M. Kaminoyama, Journal of Chemical Engineering of Japan, 40, 939-946 (2008). |
