| Search for content and authors |
Properties of the Diazo Group (-N=N-) Modified by Oxidation |
| Krzysztof Ejsmont 1, Tadeusz M. Krygowski 2, Michal K. Cyranski 2, Beata T. Stepien 2 |
|
1. Opole University, Institute of Chemistry (OU), Oleska 48, Opole 45-052, Poland |
| Abstract |
The oxidation of the diazo group (-N=N-) leads to the azoxy and azodioxy compounds [1] as shown below: 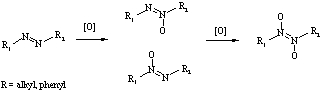 The functional groups -NN(O)-, -(O)NN- and -N(O)N(O)- differ in their properties as substituents. To quantify the extent of the changes of electronic structure of the diazo group upon oxidation we have applied a method of estimation the resonance substituent constance (σ- values), which based on the variation of the aromatic character of 8-substituted heptafulvene [2]. While the electronic character of the -NN(O)-, -(O)NN- groups is rather similar to the diazo group (σ- = 1.08, 1.00 and 1.02, respectively), the azodioxy group is significantly more electron-accepting substituent (with σ- = 1.20). The analysis is based on the ab initio optimized geometries at DFT B3LYP/6-311+G** level of theory. Since the diazo- and azoxy- groups are isoelectronic with the nitroso and nitro groups, the comparative study of their influence on π-electron delocalization of benzene was performed. [1] A. F. Wells, Structural Inorganic Chemistry, Oxford University Press, 1984, ch. 18.
|
| Legal notice |
|
| Related papers |
Presentation: poster at 18th Conference on Physical Organic Chemistry, Posters, by Krzysztof EjsmontSee On-line Journal of 18th Conference on Physical Organic Chemistry Submitted: 2006-06-06 12:32 Revised: 2009-06-07 00:44 |