| Search for content and authors |
Trifluoromethylation of bicyclic lactones leading to a new type of fluorinated morpholinols |
| Grzegorz Mlostoń , Aneta Wróblewska |
|
University of Lodz, Department of Organic and Applied Chemistry (UŁ), Tamka 12, Łódź 91-403, Poland |
| Abstract |
| Differently substituted morpholin-2-ols are of significant importance for the preparation of diverse drugs [1], but to the best of our knowledge their fluorinated analogues have not been reported, yet. On the other hand, it is well established, that introduction of fluorine atom or a perfluoroalkyl substituent, especially trifluoromethyl group CF3, results in substantial modification of physico-chemical properties and biological activities of organic compounds [2]. Therefore, elaboration of new protocols for synthesis of fluorinated compunds is in focus of medicinal chemistry [3].
The goal of the present study was the synthesis of bis-heterocyclic compounds starting with L-prolinol and arylglyoxals 1. The initial formation of bis-heterocycles 2 was in line with the results of a similar study already described in the literature [4]. However, we found that they are labile compounds and smoothly undergo diastereoselective isomerisation in the presence of an acidic catalyst yielding bicyclic morpholinones 3. 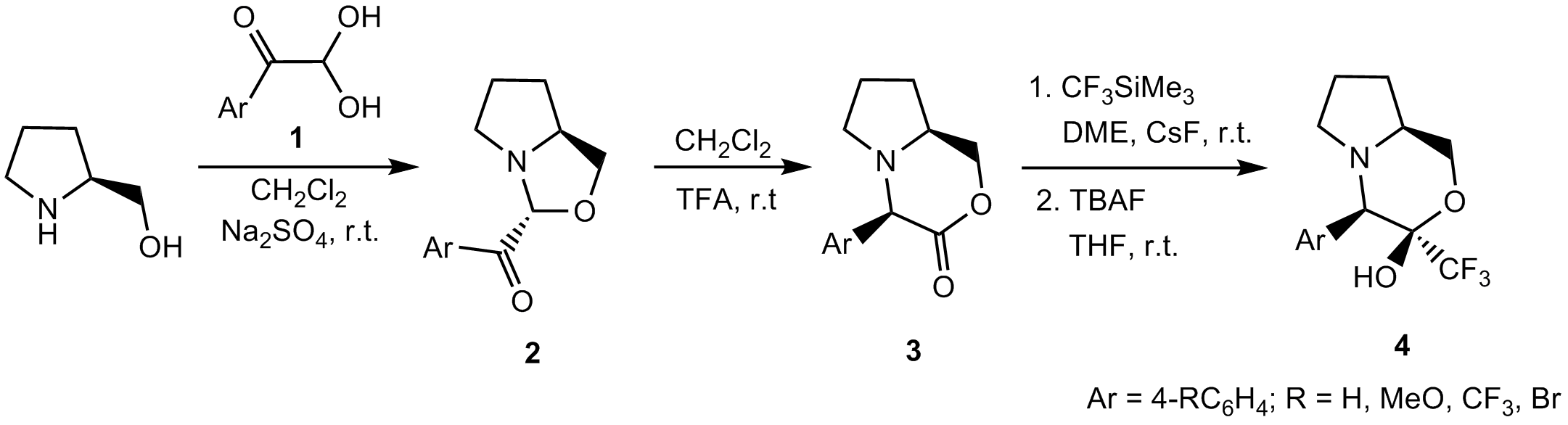
The latter were used for nucleophilic trifluoromethylation reaction via treatment with (trifluoromethyl)trimethylsilane TMS-CF3 (Ruppert-Prakash reagent) in the presence of cesum fluoride CsF as a catalyst. Trifluoromethylated morpholinols 4 with trans orientation of the CF3 and Ar groups were formed in a complete stereoselective manner. The mechanism of the aroyl 1,2-migration leading to the formation of bicyclic morpholinones 3 and the influence of the para-substituent on the rate of isomerisation will be discussed. References: [1] a) B. N. Balasubramanian, et al., Bioorg. Med. Chem. Lett., 2003, 13, 1419; b) A. P. Kourounakis, A. N. Matralis, A. Nitikatis, Bioorg. Med. Chem., 2010, 18, 7402. [2] a) V. A. Petrov, Ed., Fluorinated Heterocylic Compounds: Synthesis, Chemistry, and Applications, J. Wiley & Sons, Inc., Hoboken, NJ, 2009; b) V. G. Nenajdenko, Ed., Fluorine in Heterocyclic Chemistry, Springer Verlag, Berlin, 2013; c) G. Mlostoń, E. Obijalska, H. Heimgartner, J. Fluorine Chem., 2010, 131, 829 (and references cited therein). [3] J.-P. Begue, D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley & Sons, Hoboken, NJ. 2008. [4] Y. Ukaji, K. Yamamoto, M. Fukui, T. Fujisawa, Tetrahedron Lett., 1991, 32, 2919.
|
| Legal notice |
|
| Related papers |
Presentation: Poster at IX Multidyscyplinarna Konferencja Nauki o Leku, by Grzegorz MlostońSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-14 17:15 Revised: 2014-05-02 13:11 |