| Search for content and authors |
On the border line of concerted and non-concerted mechanisms in the [2+3]-cycloaddition reactions |
| Grzegorz Mlostoń |
|
University of Lodz, Department of Organic and Applied Chemistry (UŁ), Tamka 12, Łódź 91-403, Poland |
| Abstract |
The [2+3]-cycloaddition reactions, known also as 'Huisgen reactions', relate to the interaction of a 1,3-dipole molecule with a dipolarophile and in typical cases they result in the formation of a five-membered cycloadducts. It is well known, that they are isoelectronic with the Diels-Alder reactions ([2+4]-cycloadditions) [1]. Both types of cycloaddition reactions are of fundamental importance for the synthesis of natural products and biologically active compounds, including drugs [2]. According to classical presentations, mechanisms of these reactions are interpreted as concerted processes, which lead to the formation of five-, and six-membered products, respectively, in a stereoselective/stereospecific manner [1]. In recent three decades diverse [2+3]-cycloadditions with relatively less known thiocarbonyl ylides of type 1, classified as 'S-centered', electron rich 1,3-dipoles, have extensively been studied. The in situ generated 1,3-dipoles of type 1 react smoothly with electron deficient dipolarophiles, e.g. tetracyanoethylene (TCNE), 1,2-bis(trifluoromethyl)-1,2-dicyanoethylene (BTE) or dimethyl dicyanofumarate (DCFM), following the non-concerted (stepwise) mechanism. The zwitterionic intermediate formed therby undergoes competitive 1,5- or 1,7-cyclisation, leading to the expected tetrahydrothiophene derivatives 2 and seven-membered products, like keteneimines of type 3, respectively (Scheme) [3]. 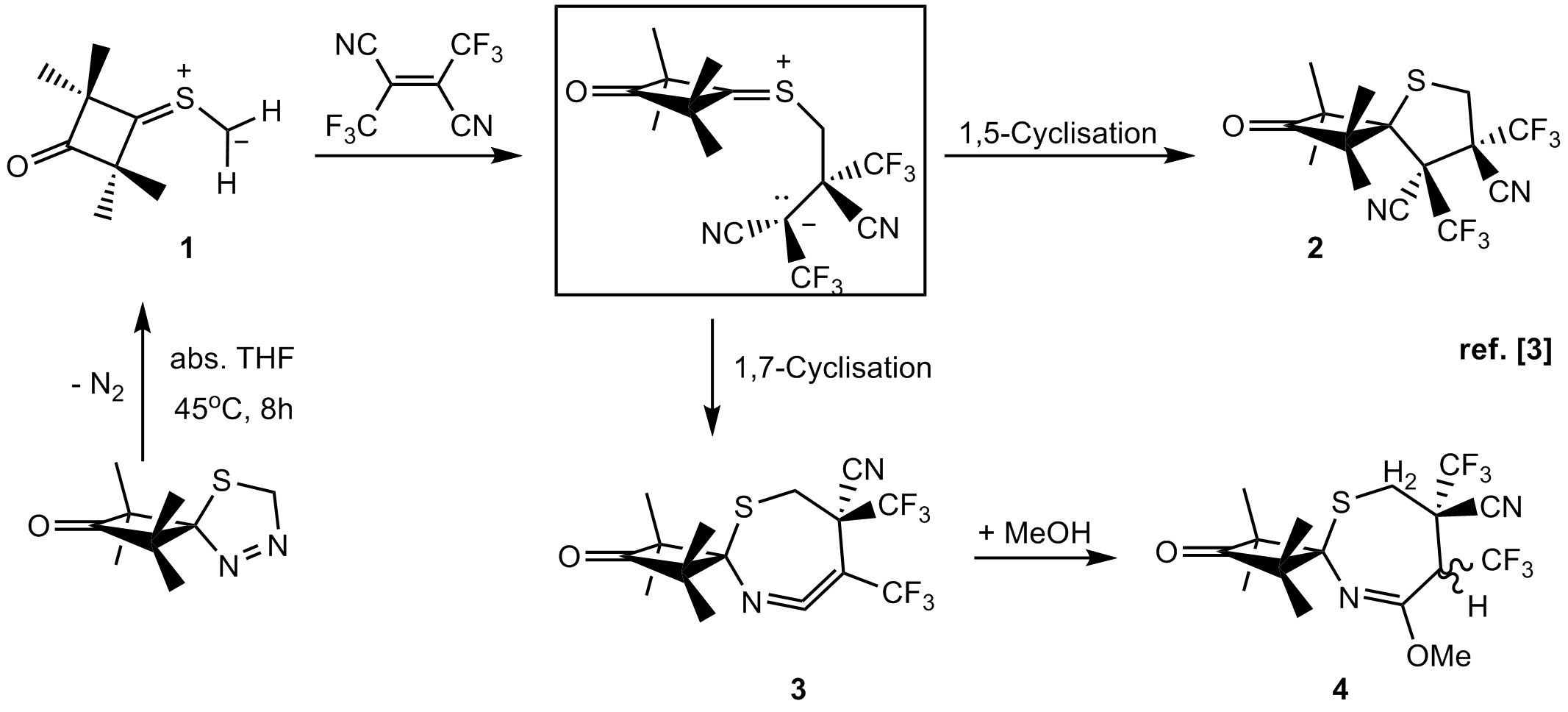 Scheme. The non-concerted pathway for the reaction of sterically crowded thiocarbonyl ylide 1 with 1,2-bis(trifluoromethyl)-1,2-dicyanoethylene. Scheme. The non-concerted pathway for the reaction of sterically crowded thiocarbonyl ylide 1 with 1,2-bis(trifluoromethyl)-1,2-dicyanoethylene.
Another examples of stepwise 1,3-dipolar cycloadditions ([2+3]-cycloadditions) with cyclic nitrones, derived from imidazole [4], as well as with azomethine ylides [5] generated via thermal ring opening of aziridines, will be discussed. Acknowledgement: Author acknowledges the National Science Center (PL-Cracow) for a generous support (Grant Maestro-3, Dec-2012/06/A/ST-5/00219). References: [1] a) R. Huisgen, Angew. Chem. Int. Ed., 1963, 2, 565; b) R. Huisgen, G. Mlostoń, in Modern Problems of Organic Chemistry, 2004, 14, 23. [2] a) C. Najera, J. M. Sansano, Org. Biomol. Chem., 2009, 7, 4567; b) G. Muncipinto, Cycloaddition Reactions in Diversity-Oriented Synthesis: Basics and Applications in Organic Synthesis, Drug Discovery, and Chemical Biology (Ed. A. Trabocchi), 2013, John Wiley & Sons, Inc., Hoboken, NJ, USA. [3] a) R. Huisgen, G. Mloston, E. Langhals, Helv. Chim. Acta, 2001, 84, 1805; b) R. Huisgen, G. Mlostoń, E. Langhals, T. Oshima, Helv. Chim. Acta, 2002, 85, 2668; c) R. Huisgen, G. Mlostoń, H. Giera, E. Langhals, Tetrahedron, 2002, 58, 507. [4] a) G. Mloston, T. Gendek, H. Heimgartner, Helv. Chim. Acta, 1998, 81, 1585; b) G. Mlostoń, M. Jasiński, D. Rygielska, H. Heimgartner, Heterocycles, 2011, 83, 765. [5] A. E. Khlebnikov, A. S. Konev, A. A. Virtsev, D. S. Yufit, G. Mlostoń, H. Heimgartner, Helv. Chim. Acta, 2014, 97, in press.
|
| Legal notice |
|
| Related papers |
Presentation: Invited oral at IX Multidyscyplinarna Konferencja Nauki o Leku, by Grzegorz MlostońSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-14 16:09 Revised: 2014-05-02 13:09 |