| Search for content and authors |
Thermodynamic stabilities of two types of quasi-liquid layers on basal faces of ice crystals |
| Harutoshi Asakawa , Gen Sazaki , Ken Nagashima , Shunichi Nakatubo , Yoshinori Furukawa |
|
Institute of Low Temperature Science, Hokkaido University (ILTS), N19-W8, Kita-ku, Sapporo 060-0819, Japan |
| Abstract |
Recently, we have succeeded in the directly observing 0.37-nm-thick elementary steps and their dynamic behavior on ice crystal surfaces1, by laser confocal microscopy combined with differential interference contrast microscopy (LCM-DIM) 2. By LCM-DIM, we found that two types of quasai-liquid layer (QLL) phases, which respectively exhibit a liquid-drop shape (α-QLLs) and a thin-layer shape (β-QLLs), appear on basal faces of ice crystals just below the melting temperature (0°C)3. The different morphologies of α- and β-QLLs strongly suggest that α- and β-QLLs have different structures. In this study, to reveal the differences in α- and β-QLLs, we examined thermodynamic stabilities of α- and β-QLLs. Sample ice crystals for the observation and other ice crystals for the supply of water vapor were prepared in an air-tight observation chamber. The temperatures of the sample and source ice crystals were controlled separately. The temperature of the sample ice determined the growth temperature Tsample of the sample ice and water vapor pressure Pe formed by the evaporation of the sample ice. The temperature of the source ice, whose volume was significantly larger than that of the sample ice, controlled water vapor pressure PH2O inside the observation chamber. We directly observed the lateral growth of elementary steps on basal faces, and then determined equilibrium ice-vapor pressure at which step velocity became zero. Plots of Vstep = 0 (open circles) in Fig.1 represent the results.We also measured the temporal changes in the sizes of α- and β-QLLs, and then determined the critical water vapor pressures of α- and β-QLLs at which their size remained constant. Plots of α- and β-QLLs (open squares and rhombus) in Fig.1 respectively summarized the results. As shown in Fig.1, the critical pressures of both QLLs are significantly higher than the equilibrium pressure between ice and vapor. This result clearly demonstrates that both QLLs are thermodynamically less stable than solid ice. Hence, we concluded that the appearances of both QLLs are due to the condensation of supersaturated water molecules on basal faces rather than the melt of most stable ice crystals. The picture of QLLs obtained in this study is quite different from the long-standing conventional picture in which a QLL phase has been considered as a thermodynamically stable phase4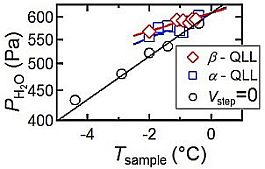
Fig. 1. Apparent phase diagram of α- and β-QLLs. Reference 1. Sazaki, G. et al., Proc. Natl. Acad. Sci. USA 2010, 107, 19702-19707. 2. Sazaki, G. et al., J. Cryst. Growth. 2004, 262, 536-542. 3. Sazaki, G. et al., Proc. Natl. Acad. Sci. USA 2012, 109, 1052-1055. 4. Kuroda, T. & Lacmann, R. J. Cryst Growth, 1982, 56 189-205. |
| Legal notice |
|
| Related papers |
Presentation: Poster at 15th Summer School on Crystal Growth - ISSCG-15, by Harutoshi AsakawaSee On-line Journal of 15th Summer School on Crystal Growth - ISSCG-15 Submitted: 2013-05-08 11:54 Revised: 2013-05-09 15:14 |