| Search for content and authors |
The comparison of immobilization for glucose oxidase and horseradish peroxidase on titania nanotube surface |
| Katarzyna Arkusz , Agnieszka Kaczmarek , Jowita Loin , Elzbieta Krasicka-Cydzik |
|
University of Zielona Gora, Podgorna 50, Zielona Góra 65-246, Poland |
| Abstract |
A growing interest in using biosensors as a modern tool in the diagnosis and treatment of various diseases has been observed. Due to the use of an increasing number of detection techniques and design concept of biosensors, the range of materials used to construct sensors is expanding. Titania nanotubes (TNT) are being investigated and developed intensively due to their advantageous qualities ie. good electrical properties, high surface area, excellent biocompatibility and controllable shaping. 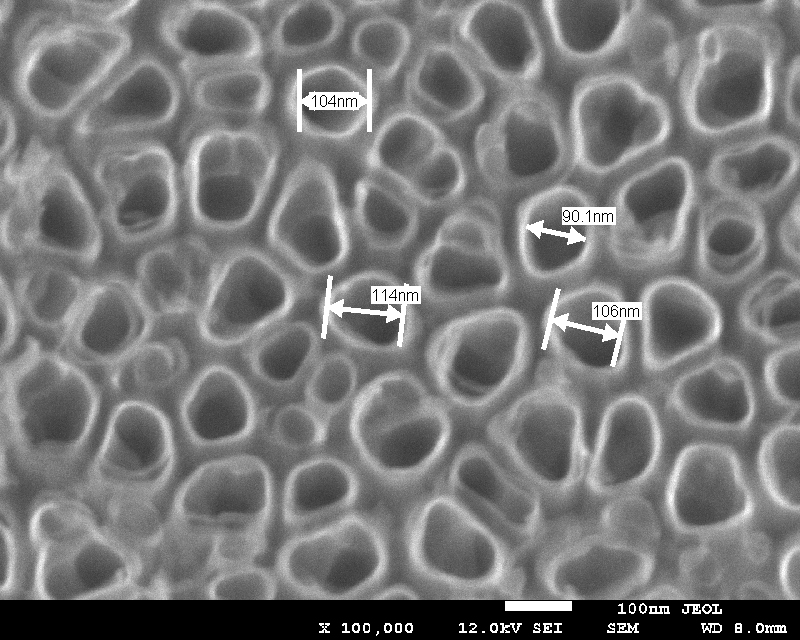
Figure 1. Surface morphology of anodic titania nanotubes formed on titanium in 1 M H3PO4 with 0.3% wt. HF Significant changes in the flow of current after binding the biomolecules to nanotubes are caused by the direct proximity of the surface. The load and the conformation changing the molecule binding to these structures gives a large effect. This is why the selection of biomarker and the method of its immobilization is so important. These studies compared the effect of immobilization method (dip or drop coating) and the influence of the immobilization time (24h and 48h) on the biosensor response in case of two model enzymes : the glucose oxidase (GOx) and horseradish peroxide (HRP) used to detect glucose or hydrogen peroxide (H2O2) in the PBS solution. The biological components covering the surface of the electrodes generate electrochemical response, making them the efficient and sensitive biosensors. Firstly, using a matrix built of TiO2 nanotubes (TNT) covered with enzymes as the platforms of the 3rd generation biosensor was assumed. The layer of TiO2 nanotubes on titanium surface was obtained by anodization method and characterized using scanning microscopy. The material was annealed in a vacuum at 450ºC, and then glucose oxidase enzyme or horseradish peroxide were immobilized on its surface. The biosensor substrate was immersed in the enzyme solution for 24 and 48 hours. To check the biosensor response and to detect the presence of glucose or hydrogen peroxide in the PBS solution the cyclic voltammetry (CV) was used. In both cases the results were not satisfactory and the use of mediators was necessary. After using potassium ferricyanide as a mediator, an electric signal responsible for the process of catalytic oxidation of glucose was obtained. The analogous studies with the use of horseradish peroxidase enzyme and thionine acetate as a mediator were carried out giving the evidence of electrical response due to H2O2 presence in the PBS solution.TNT platform for the 3rd generation biosensor was considered.
Figure 2. Cyclic voltamperometry measurements of : a) glucose biosensor – glucose oxidase GOx in PBS (pH 7.4), 0.1 M KCl and 5mM glucose, b) hydrogen peroxide biosensor - horseradish peroxidase HRP in PBS (pH 7.4), 0.1 M KCl and 100ml 30% H2O2 The studies allowed to elaborate the method of electrodes preparation of the 2nd generation biosensors based on titania nanotubes and will allow to optimize their structure as the efficient and simple biosensors. |
| Legal notice |
|
| Related papers |
Presentation: Short communication at SMCBS'2011 International Workshop, by Katarzyna ArkuszSee On-line Journal of SMCBS'2011 International Workshop Submitted: 2011-08-30 14:11 Revised: 2011-10-27 20:13 |