The growth of single crystals has been developed over decades to meet the needs for basic research and applications in many different areas. On this aspect, fluorides materials have shown a continuous development on research and technological uses as dosimeters, x-ray monochromators and mainly as optical devices, such as optical windows and laser hosts. The BaY2F8 compound has recently been the subject of several studies, especially regarding the spectroscopy of rare earth (RE) doped crystals for determination of their potential for new compact diode pumped laser systems. Although numerous reports in the literature deal with the laser properties of RE-doped BaY2F8 crystals, only a few of them study their preparation in details. In the present work we describe our results on the synthesis from constituent compounds, phase diagram study and growth process of pure and RE-doped BaY2F8 crystals. The basic constituents, BaF2 and the YF3, and the rare earth dopants (REF3) are available from different commercial suppliers with high purity. However, better results concerning the elimination of moisture and oxygen contamination can be obtained from fluorides prepared in laboratory from commercial Y2O3 (or RE2O3) and BaCO3 by hydrofluorination [1]. In this work an open boat with the oxides (or carbonates) was placed inside a Pt chamber and heated up to the reaction temperature (~850oC). An HF/Ar flow was kept constant for 2-3 hours. The system was cooled to room temperature and before opening the chamber was rinsed with pure Ar to eliminate traces of HF.
The phase equilibrium relations for BaF2 - YF3 system were investigated with the particular intent of better understanding the growth process of BaY2F8 crystals. Samples of different compositions of BaF2 and YF3 previously synthesized were melted under HF atmosphere. The obtained samples were subjected to thermal analysis (differential thermal analysis, differential scanning calorimetry and thermogravimetry). The samples have also been subjected to analysis via X-ray powder diffraction and quantitative calculations of phase concentrations using the Rietveld method were performed. The obtained results were compared with the phase diagrams found in the literature [2,3].
DTA measurements were accomplished in a simultaneous TG/DTA system from TA Instruments, model SDT 2960. As the fluorides are known to be highly sensitive to moisture, fast heating rates (40oC/min) were used to minimize contamination together with a constant flow of high purity inert gas during measurements. DSC/TG measurements were performed with a Netzsch STA 449C-Jupiter model using heating/cooling rates of 10 K/min. In this case the sample powders were evacuated prior to heating to remove adsorbed water. During the measurements a flow of Ar was maintained. Usually DSC curves from the second heating run were used for analysis.
Different compositions were observed to the right and to the left of the 1:2 composition (BaY2F8 phase), respectively. Figure 1 shows DSC curve obtained for 1:2 composition showing the melting peak in detail. More than one thermal effect seems to occur in a very short temperature range. Tkachenko et al [3] proposed that a polymorphous transition from a β-BaY2F8 to an α-BaY2F8 structure associated with small atomic displacements occurs. A phase transformation may occur some degrees bellow the BaY2F8 melting point, nevertheless, as it occurs at sufficiently high temperature defect healing can occur and single crystals of good quality can be grown from the melt. The second curve also showed in Figure 1, corresponds to one of the eutectic compositions at the phase diagram.
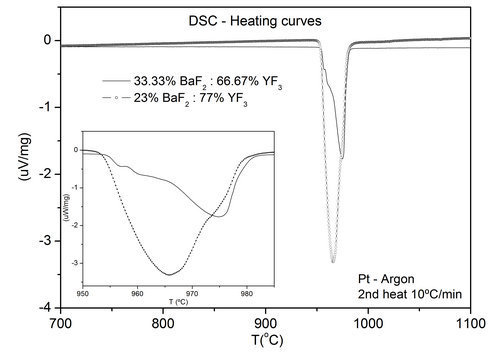
Figure 1. DSC heating curves obtained from second heating of two different compositions of YF3 and BaF2.
Rietveld quantitative phase analyses were done using experimental CuKα X-ray powder patterns obtained with the same samples prepared to thermal analyses. Rietveld analyses were performed by using the program DBWS-9807a [4]. The results are listed in Table 1. Pure and Nd, Er, Tb single doped and Nd:Dy co-doped crystals were obtained by zone melting (ZM) under fluorinating atmosphere. The choice of ZM was done taking into account the simplicity of the process when compared to other growth methods from the melt used in the production of small and high purity crystals as those required by diode pumping laser systems. ZM experiments were carried out in vitreous-carbon crucibles inserted in a platinum tubular reactor under HF flow. The total length of the ingots was about 290 mm and the length of the liquid zone was 26 mm. Experiments were performed using a zone speed rate of 2mm/h. The resulting crystals were characterized by spectroscopy and x-ray diffraction. The absorption spectra showed no evidence of incorporation of optically active impurities (except dopants) that could compromise their optical performance. The concentration of rare earth ions along the obtained zone melted ingots was measured by EDX showing uniform distribution.
![]()
(This work was supported by CNPq and CAPES).
References
[1] S.L. Baldochi, S.P. Morato, Fluoride bulk crystals growth. In: K.H.J. Buschow, R.W. Cahn, M.C. Flemings, B. Ilschner, E.J. Kramer, S. Mahajan (eds.), Encyclopedia of Materials: Science and Technology (Elsevier Science Amsterdam, 2001) pp. 3200-3205.
[2] E. G. Ippolitov, A.G. Maklachko, Inorg. Mater. (USSR) (Engl. Tranl.), 6 (1), (1970) 124.
[3] N.L. Tkachenko, M. Svantner, B.P. Sobolev, Inorg. Mater. (USSR) (Engl. Tranl.), 13 (5), (1977) 693.
[4] R.A. Young, A.C. Larson and C.O. Paiva-Santos., User´s Guide to Program DBWS-9807a for Rietveld Analysis of X-ray and Neutron Powder Diffraction Patterns (Georgia Institute of Technology, Atlanta., 1999).
|