| Search for content and authors |
Aqua Magnesium(II) Phthalocyanine Bis(diethylamine) Solvate |
| Vasyl Kinzhybalo , Jan Antczak |
|
Polish Academy of Sciences, Institute of Low Temperature and Structure Research (INTiBS), Okólna 2, Wrocław 50-422, Poland |
| Abstract |
Magnesium phthalocyanine (MgPc) and its MgPcL and MgPcL2 coordination complexes (4+1 and 4+2) with N and O donor ligands deserve attention first of all because of similarity with chlorophyll, thus being its synthetic models. Due to their electrochemical properties they find application in solar energy conversion, are used as pigments in optical disks, laser printers and display devices. Particular interest in aquamagnesium phthalocyanine ('X-phase') is concerned with its solid-state intence near-IR absorption band, which origin is not completely clear up to now. Two of the works dedicated to the problem give different explanation: first assigns MgPc(H2O)2 composition to the 'X-phase' and suggests that the near-IR absorption arises from extinction coupling effects [1], second analyses crystal structures of triclinic and monoclinic modifications of MgPc(H2O), of which only triclinic one exhibits near-IR absorption. Similarity of the structures of triclinic MgPc(H2O) and β-MgPc, which also exhibits the phenomenon of near-IR absorption, suggests that the origin could be found in formation of face-to-face π-π stacked dimers [2]. 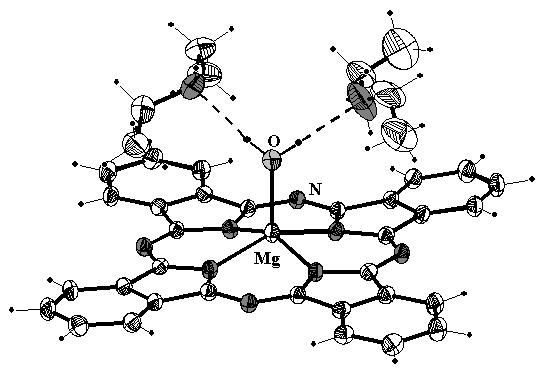
Violet crystals of the title compound were obtained by recrystallization of MgPc from diethylamine solution, sealed in glass ampoule, at 50 ºC during 12 hours. The compound is stable up to 140 ºC, where it loses simultaneously both diethylamine solvate molecules, at 195 ºC it loses water molecules and transforms to the β-MgPc. Structure of MgPc(H2O)·2Et2NH is built of MgPc(H2O) moieties, stacked in dimers by back-to-back fashion. These dimers pack together via very weak hydrogen-bond interactions of C-H...N type into infinite planes perpendicular to c-axis direction at 0 and ½ height of the unit cell. Two diethylamine moieties are in the outer coordination sphere, attached to water molecule by O-H...N H-bonds (1.94(2), 1.97(2) Å and 172(2), 167(2)º for H...N distances and O-H...N angles, respectively). N-H protons are not involved in any H-bond formation. The ethyl groups exhibit high thermal librations. Mg atom deviates from N4-isoindole plane by 0.494(2) Å, some 0.04 Å more than in two polymorphs of MgPc(H2O). The Mg-O distance (1.993(2) Å) and four Mg-N distances (2.038(2)-2.040(2) Å) are comparable with the appropriate values in the MgPc(H2O). Saucer-shaped geometry would be an expected one for 4+1 coordinated MgPc moiety, but it is considerably distorted by flattering through repulsive π-π interactions between two Pc rings, which are overlapped along isoindole-N axis. [1] A. Endo, S. Matsumoto, J. Mizugushi, J. Phys. Chem. A 1999, 103, 8193. |
| Legal notice |
|
Presentation: poster at 18th Conference on Physical Organic Chemistry, Posters, by Vasyl KinzhybaloSee On-line Journal of 18th Conference on Physical Organic Chemistry Submitted: 2006-06-01 15:31 Revised: 2009-06-07 00:44 |