| Search for content and authors |
Kinetic Study of the Benzenethiolysis of S-Methyl Aryl Thiocarbonates |
| Margarita Aliaga , Enrique A. Castro , José G. Santos |
|
Pontificia Universidad Catolica de Chile (PUC), Vicuna Mackenna 4860, Santiago 6094411, Chile |
| Abstract |
The reactions of S-methyl 4-nitrophenyl thiocarbonate (SMNPTC) and S-methyl 2,4-dinitrophenyl thiocarbonate (SMDNPTC) with a homogeneous series of benzenethiolate anions are subjected to a kinetic investigation in water, 25.0° C, ionic strength 0.2 M (KCl). Under benzenethiolate excess over the substrate all these reactions obey pseudo-first-order kinetics and are first order in the nucleophile.
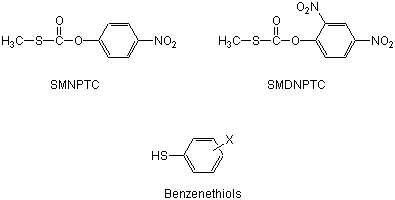
The Brönsted-type plots for the nucleophilic rate constants are linear, with slopes β = 0.55 and β = 0.70 for the reaction of SMNPTC, and SMDNPTC respectively with benzenethiols suggesting a concerted mechanism [1]. SMDNPTC is more reactive than SMNPTC toward benzenethiolate ions due to a carbonyl carbon more positively charged and a better leaving group. A comparison of these results with those for the concerted phenolysis of the same substrates indicates that benzenethiolates are better nucleophiles toward thiocarbonates than isobasic phenoxides [2]. M.A. thanks CONICYT of Chile (AT-24050119) for doctoral fellowships. [1] E. A. Castro, P. Pavez, J. G. Santos, J. Org. Chem. 2001, 66, 3129. [2] E. A. Castro, P. Pavez, J. G. Santos, J. Org. Chem. 2003, 68, 3640-3645. |
| Legal notice |
|
| Related papers |
Presentation: poster at 18th Conference on Physical Organic Chemistry, Posters, by Margarita AliagaSee On-line Journal of 18th Conference on Physical Organic Chemistry Submitted: 2006-03-23 18:13 Revised: 2009-06-07 00:44 |