| Search for content and authors |
Fluorinated carbanucleosides and acyclic nucleosides |
| Magdalena Telega , Hanna Wójtowicz-Rajchel |
|
Adam Mickiewicz University, Faculty of Chemistry, Grunwaldzka 6, Poznań 60-780, Poland |
| Abstract |
Fluorinated analogs of nucleosides find use as antiviral and anticancer agents. This is possible due to their ability to act as inhibitors of nucleotide metabolism- they are indistinguishable by active site of enzymes. Properties of fluorine atom are very important here (similar size to the hydrogen atom, large electronegativity, the possibility of replacing a hydroxyl group). 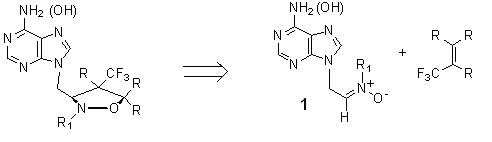
Our synthetic task was to obtain presented above nitrone (1) from the respective purine bases. These compounds would be subjected to the 1,3-dipolar cycloaddition with fluorinated olefins. Next step would be reaction of opening isoxazolidinyl ring. References: [1] Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Albert Padwa and William H. Pearson, John Wiley & Sons, 2003  |
| Legal notice |
|
| Related papers |
Presentation: Poster at IX Multidyscyplinarna Konferencja Nauki o Leku, by Magdalena TelegaSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-19 20:46 Revised: 2014-05-02 19:25 |