| Search for content and authors |
Generator/collector experiments at a single, chemically-modified electrode: introduction and general applicability |
| Gregory G. Wildgoose 1, Martin C. Henstridge 2, Richard G. Compton 2 |
|
1. University of East Anglia (UEA), Norwich NR47TJ, United Kingdom |
| Abstract |
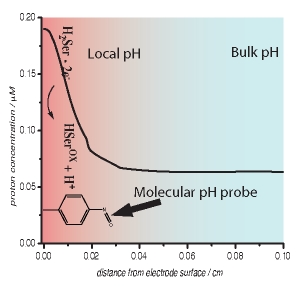
We demonstrate proof-of-concept that generator-collector experiments can be performed at a single macroelectrode and used to determine mechanistic information. The facile chemical modification of a graphite electrode with pH sensitive redox active molecules results in the formation of a single electrode generator/collector electrode system. Using the oxidation of serotonin as a model system, we demonstrate that the single generator/collector electrode is capable of measuring changes in local pH immediately adjacent to the electrode surface, i.e. within the diffusion layer, during the oxidation process. Comparison of experimental data with simple 1-D numerical simulations was used to ascertain that the serotonin oxidation mechanism in poorly buffered media initially involves the transfer of two-electrons and only one proton, in an ECE mechanism. This approach compares favorably in terms of sensitivity to traditional double electrode experiments such as the use of rotating ring-disc electrodes. Experiments were also performed with a different molecular pH probe, either dissolved in the electrolyte solution or attached to the electrode surface, in order to compare the general feasibility of this approach. The reduction of dissolved oxygen in water was used as a model system with which to compare the performance of the two approaches. The results of these experiments suggest that the single-electrode generator/collector approach is potentially readily applicable to a much wider range of electrochemical systems. |
| Legal notice |
|
Presentation: Keynote lecture at SMCBS'2009 International Workshop, by Gregory G. WildgooseSee On-line Journal of SMCBS'2009 International Workshop Submitted: 2009-09-01 14:48 Revised: 2009-10-29 15:35 |