The structures of 2,4-dioxo-1,2,3,4-tetrahydropyrido[2,3-d]pyrimi-
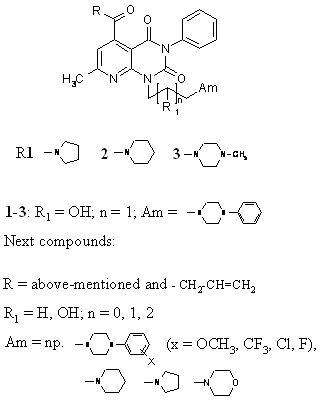
dine derivatives synthesized at the Department of Chemistry of Drugs consist of the three basic fragments: the aliphatic spacer linking two pharmacophore termini: pyrido[2,3-d]pyrimidine nucleus and N-phenylpiperazine or other cyclic amine. From our investigations in this field it follows that all above presented fragments have influence on biological activity. The findings obtained for amides of 1-[2-hydroxy-3-(4-phenyl-1-piperazinyl)propyl]-7-methyl-3-phenyl-2,4-dioxo-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-5-carboxylic acid 1-3 [1] indicate that the strength of the analgesic action depends on the structure of the amide group. All compounds 1-3 displayed in pharmacological screening strong but different antinociceptive properties in the “writhing syndrome” test (values of ED50 are as follows 1.44 (1), 13 (2), 6.1 mg/kg (3)). Having to regard to it we synthesized then series N,N-dialkyl(dialkenyl)amides [2]. All obtained substances showed also strong analgesic activity in above mentioned test (ED50=1.53-5.31 mg/kg). In the further investigations we introduced pharmacophoric substituents (Cl, F, OCH3, CF3) into the phenyl at N-4 of piperazine [3,4]. Most of them exhibited significant analgesic activity and this activity was comparable or superior than that of aspirin. Next modification concerned the structure of alkyl spacer. It was stated [5] that the prolongation of the side-alkyl chain at N-1 to C-4 and elimination of the hydroxy group weakened the analgesic properties and increased toxicity. Continuing investigations in this group of compounds we wanted to know which influence on analgesic activity and toxicity would have: 1) shortening of the alkyl linker to C-2 with simultaneous replacement of N-phenylpiperazinyl substituent by other cyclic amines (piperidine, pyrrolidine...); 2) introduction of pharmacophoric substituents (CH3, CF3) into the phenyl at N-4 of piperazine in above mentioned butyl derivatives.
[1]. H.Śladowska et al. Farmaco 54 (1999) 773-779
[2]. H.Śladowska et al. Farmaco 58 (2003) 25-32
[3]. A.Sabiniarz et al. Acta Polon.Pharm.-Drug Res. 64 (2007) 369-376
[4]. A.Sabiniarz et al. in press
[5]. H.Śladowska et al. Farmaco 55 (2000) 6-12 |