| Search for content and authors |
Synthesis, structure and biological activity of 1-[(imidazolidin-2-yl)imino]azoles* |
| Franciszek Sączewski 1, Anita Kornicka 1, Apolonia Rybczyńska 2, Alan L. Hudson 3, Shu S. Miao 3, Maria Gdaniec 4, Konrad Boblewski 2, Artur Lehmann 2 |
|
1. Medical University of Gdańsk, Department of Chemical Technology of Drugs, Al. Gen. J. Hallera 107, Gdańsk 80-416, Poland |
| Abstract |
In connection with our previous studies on novel imidazoline compounds [1-3] with alpha2-adrenoceptor and/or imidazoline I1/I2 receptor affinity, we have prepared a series of new indazole A - B, benzimidazole C and benzotriazole D derivatives bearing (imidazolidin-2-yl)imino moiety at position 1 or 2.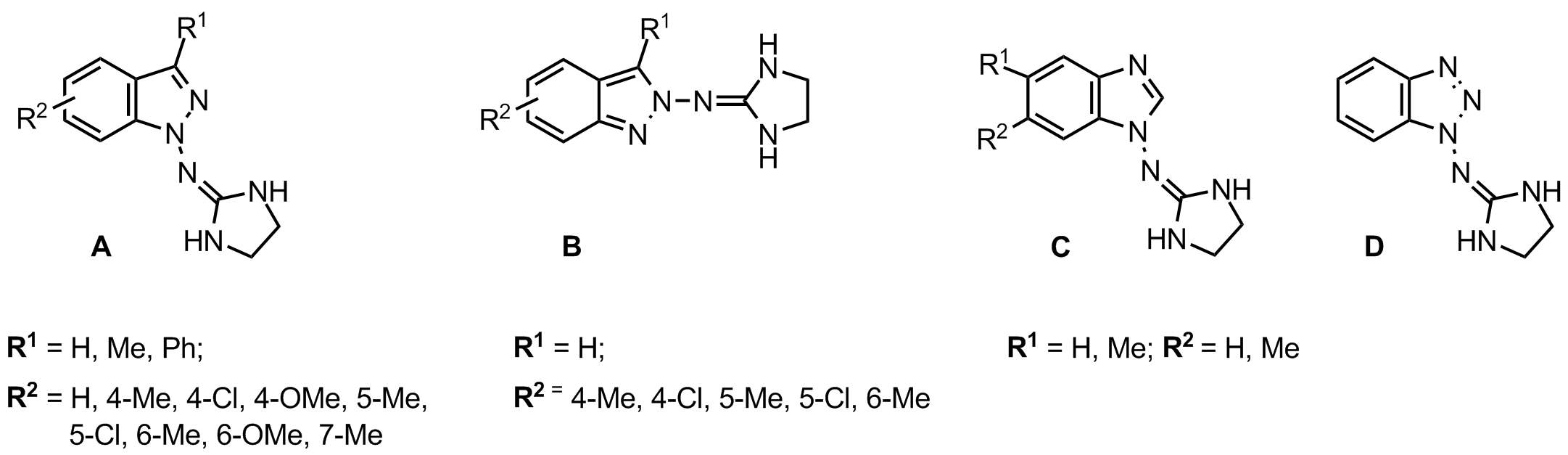
The in vitro assays involving investigation of the affinity and selectivity of the newly synthesized imidazoline ligands for alpha2-adrenoceptors, imidazoline I1 and imidazoline I2 binding sites showed very low or no affinity for imidazoline I2 receptors. 1-[(Imidazolidin-2-yl)imino]indazole (marsanidine), the most active agent at alpha2-adrenoceptors (Ki = 14 nM), displayed a very high alpha2/I1 selectivity ratio = 3879. *This research was supported by the Polish Ministry of Science and Higher Education (Grant N40500532/0458). Sączewski F., Kornicka A., Rybczyńska A., Hudson A.L., Miao S.S., Gdaniec M., Boblewski K., Lehmann A. 1[(Imidazolidin-2-yl)imino]indazole (Marsanidine) – highly alpha2/I1 selective agonist: synthesis, X-ray structure and biological activity - accepted for publication in J. Med. Chem. 2008; Sączewski F., Kornicka A., Rybczyńska A., Hudson A.L. Nowe pochodne 1-[(imidazolidyn-2-ylo)imino]indazolu i sposób ich otrzymywania. Patent Application P 383955. References: |
| Legal notice |
|
| Related papers |
Presentation: Poster at VI Multidyscyplinarna Konferencja Nauki o Leku, by Anita KornickaSee On-line Journal of VI Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2008-03-11 15:55 Revised: 2009-06-07 00:48 |