| Search for content and authors |
Application of 1-amino sugar in synthesis of glycoconjugates and determination of their biological activity toward β-1,4-galactosyltransferase |
| Roman Komor , Gabriela Pastuch-Gawołek , Mateusz Pleśniak |
|
Silesian University of Technology, Department of Organic Chem., Bioorganic Chem. and Biotechnol., Krzywoustego 4, Gliwice 44-100, Poland |
| Abstract |
Glycosyltranferases are enzymes responsible for the formation of the glycosidic bond in living system. They transfer the sugar unit from a nucleoside diphosphate sugar (NDP-sugar e.g. UDP-glucose) to a specific hydroxyl- or amino- group of the acceptor substrate which is usually other sugar but nucleic acids, proteins, lipids and other small molecules are also encountered [1]. Modification of many structures through addition of sugars unit can have a significant impact on their physical, chemical or biological properties. Current research confirms that cell surface glycoconjugates have pivotal functions in various cellular recognition systems involving cell differentiation, development, inflammation, immune response, bacterial/viral infections, and many other intercellular communications [2]. In recent years increased attention toward GTs inhibitors has emerged due to key role of these enzymes in biological synthesis of glycoconjugates and polysaccharides. Development of new selective inhibitors is of great importance in dealing with bacterial [3] and fungal diseases [4]. Analogues of NDP-sugar can have an important role as new drug candidates. Our recent research led to analogues of GTs natural substrates in which diphosphate moiety was replaced with amide unit connecting carbohydrate to cyclic or acyclic nucleoside part. Alternative route includes 1,2,3-triazole linkage installed by dipolar cycloaddition. 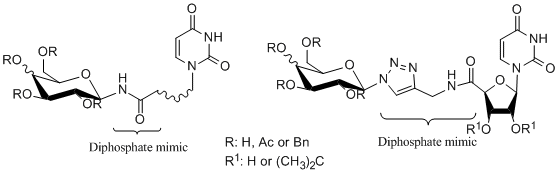 Acknowledgement Research studies part-financed by the European Union within the European Regional Development Fund (POIG.01.01.02-14-102/09). Roman Komor received a scholarship under the project DoktoRIS - Scholarship Program for Innovative Silesia. References: [1] L. L. Lairson, B. Henrissat, G. J. Davies, S. G. Withers, Annu. Rev. Biochem., 2008, 77, 521-555. [2] K. Hosoguchi, T. Maeda, J. Furukawa, Y. Shinohara, H. Hinou, M. Sekiguchi, H. Togame, H. Takemoto, H. Kondo, S.-I. Nishimura, J. Med. Chem., 2010, 53, 5607-5619. [3] S. Berg, D. Kaur, M. Jackson, P. J. Brennan, Glycobiology, 2007, 17, 35-56. [4] J. B. Behr, A. Gainvors-Claisse, A. Belarbi, Nat. Prod. Res., 2007, 21, 76-82. |
| Legal notice |
|
| Related papers |
Presentation: Poster at IX Multidyscyplinarna Konferencja Nauki o Leku, by Gabriela Pastuch-GawołekSee On-line Journal of IX Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2014-03-11 18:37 Revised: 2014-05-02 17:10 |