| Search for content and authors |
Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity. Part 1. |
| Franciszek Herold 1, Andrzej Chodkowski 1, Łukasz Izbicki 1, Jerzy Kleps 1, Marek Król 1, Gabriel Nowak 2,3, Katarzyna Stachowicz 2, Małgorzata Dybała 3, Agata Siwek 3 |
|
1. Medical University of Warsaw, Department of Drug Technology, Banacha 1a, Warszawa 02-097, Poland |
| Abstract |
One approach toward new antidepressants with better clinical parameters is the synthesis of compounds with dual SERT inhibitor/5-HT1A activity [1]. Among many concepts in this field, the idea of incorporating a 5-HT1A antagonist component into the structure of an SSRI is thought to be the most advantageous. However, there is a concern that the use of 5-HT1A receptor antagonist without discriminating between its pre- and postsynaptic activity could cancel the benefits of enhancing presinaptically the 5-HT function. Therefore an alternative method is the design of compounds with dual 5-HT reuptake blockade/5-HT1A receptor agonist action, which could desensitize presynaptic 5-HT1A receptors and postinaptically enhance serotonin transmission [2-4]. 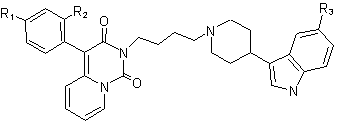
R1, R2, R3: 7a: H, H, OCH3; 7b: H, OCH3, H; 7c: H, OCH3, OCH3; 7d: H, Cl, H; 7e: H, Cl, OCH3; 7f: F, H, OCH3; 7g: H, F, H; 7h: H, F, OCH3; 7i: H, H, H; 7j: F, H, H; 7k: H, CH3, H; 7l: H, CH3, OCH3; [1] Spinks D., Spinks G.; Curr. Med. Chem. 9, 799-810 (2002)[2] Romero L., Celada P., Martin-Ruiz R., Diaz-Mataix L., Mourelle M., Delgadillo J., Hervas I., Artigas F.; Neuropsychopharmacology 28, 445-456 (2003) [3] Zhou D., Harrison B., Shah U., Andree T., Hornby G., Scerni R., Schechter L., Smith D., Sullivan K., Mewshaw R., Bioorg. Med. Chem. Lett. 16, 1338–1341 (2006) [4] Rocco V., Spinazze P., Kohn T., Honigschmidt N., Nelson D., Wainscott D., Ahmad L., Shaw J., Threlkeld P., Wong D., Takeuchi K., Bioorg. Med. Chem. Lett. 14, 2653–2656 (2004) |
| Legal notice |
|
| Related papers |
Presentation: Poster at VI Multidyscyplinarna Konferencja Nauki o Leku, by Łukasz IzbickiSee On-line Journal of VI Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2008-03-13 11:53 Revised: 2009-06-07 00:48 |