| Search for content and authors |
Tautomerism of Acridin-9-amines |
| Jerzy Błażejowski 1, Youssif Ebead 1, Karol Krzymiński 1, Artur Sikorski 1, Agnieszka Wróblewska 1, Alexander D. Roshal 2, Andrey O. Doroshenko 2 |
|
1. University of Gdańsk, Faculty of Chemistry, J. Sobieskiego 18, Gdańsk 80-952, Poland |
| Abstract |
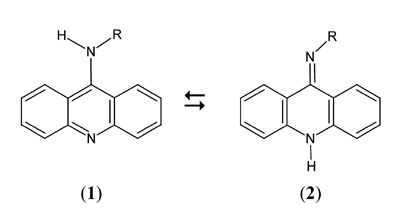
Acridin-9-amines and their derivatives substituted at the exocyclic nitrogen atom can co-exist in amino (1) and imino (2) tautomeric forms. Electron-donating substituents (R) cause the amino form to be more stable, but electron-attracting ones cause the imino tautomer to prevail. Crystals of acridin-9-amines substituted with -t-Bu or -CH2CH2Cl at the exocyclic nitrogen atom consist of molecules of the amino tautomer, but those with -OCH3 or -COCl3 - contain molecules of the imino form. NMR investigations have shown that if R is -OCH3 or -NH2, the respective acridin-9-amines occur in the imino tautomeric form in various media. When R is -CH2CH2Cl or 5-methylpyridin-2-yl, the preference for a particular tautomeric form depends on the properties of the medium. The results of stationary absorption and fluorescence, time-resolved fluorescence investigations and computational studies have demonstrated that if -CH2CH2Cl is a substituent, it is mainly the imino form that absorbs radiation, whereas the amino form fluoresces. When the substituent is 5-methylpyridin-2-yl, the absorbing form is mainly the amino tautomer, and the emitting form is the imino one. As tautomeric forms of the latter two compounds that absorb radiation do not emit it, is probable that H-atom transfer takes place in the excited state. The spectral properties of these acridin-9-amines, both NMR and UV-Vis, thus depend markedly on the properties of the solvent, which makes them potent indicators of the properties of liquid phases. On the other hand, the tautomerism of acridin-9-amines is an interesting subject for investigating the fundamental behavior of chemical systems. (Study supported by state funds for scientific research under DS/8000-4-0026-6) |
| Legal notice |
|
| Related papers |
Presentation: oral at 18th Conference on Physical Organic Chemistry, Symposium 1, by Jerzy BłażejowskiSee On-line Journal of 18th Conference on Physical Organic Chemistry Submitted: 2006-05-30 11:21 Revised: 2009-06-07 00:44 |