| Search for content and authors |
SYNTHESIS AND ANTICONVULSANT ACTIVITY OF NOVEL N-PYRIDINE-2-YL AND N-AMINOPHENYL DERIVATIVES OF 3,3-DIALKYL-PYRROLIDINE-2,5-DIONES |
| Krzysztof Kamiński , Jolanta Obniska |
|
Jagiellonian University, Medical College, Department of Pharmaceutical Chemistry, Medyczna 9, Kraków 30-688, Poland |
| Abstract |
In the recent years we have synthesized a great number of spirosuccinimides with anticonvulsant activity by changing substituents at the imide nitrogen atom [1, 2]. In the current study a new series of N-pyridine-2-yl and N-aminophenyl derivatives of 3,3-dialkyl-pyrrolidine-2,5-diones were synthesized. These molecules were designed as analogues of 3-ethyl-3-methyl-pyrrolidine-2,5-dione (ethosuximide), to evaluate the influence of such modifications on anticonvulsant activity. 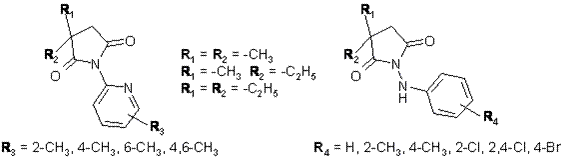 The compounds were evaluated for their anticonvulsant and neurotoxic properties within the Antiepileptic Drug Development (ADD) Program by testing procedures, which have been described earlier [3]. One of the current approaches in rational drug design is to estimate the lipophilic character of the molecules. Therefore for all compounds the lipophilicity was determined by use of the Pallas 3.1 computer program. The results obtained provided a good basis for the evaluation of structure-lipophilicity relationships as well as the correlation between clogP values and anticonvulsant activity. References: 1. Obniska J., Dzierżawska-Majewska A., Zagórska A., Zajdel P., Karolak-Wojciechowska J.; Il Farmaco 60 (2005) 529-539. 2. Kamiński K., Obniska J., Zagórska A., Maciąg D.; Arch. Pharm. in press (2006). 3. Kupferberg H.J.; Epilepsia 30 (suppl.) (1989) 51-56. |
| Legal notice |
|
| Related papers |
Presentation: Poster at V Multidyscyplinarna Konferencja Nauki o Leku, by Krzysztof KamińskiSee On-line Journal of V Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2006-02-21 11:41 Revised: 2009-06-07 00:44 |