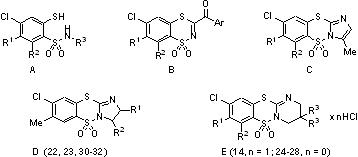
Variously substituted 2-mercaptobenzenesulfonamides (A) and 3-aroyl-1,1-dioxo-1,4,2-
benzodithiaznes (B) were synthesized in our laboratories and described as a novel class of potent HIV-1 integrase inhibitors [1,2]. Recently, we have found that the compound of type C (R1 = Me, R2 = H) showed also remarkable anti-HIV activity with 50% effective concentration EC50 value of 0.94 μM and no significant cytotoxicity at 200.0 μM [3].
The above findings prompted us to develop new methodologies for the syntheses of the compounds depicted as D and E. The syntheses of the target compounds were achieved by convenient three or four step procedures starting from the corresponding 4-chloro-3-methylthio-1,4,2-benzodithiazine 1,1-dioxides.
The in vitro anti-HIV activity of compounds 14, 22-25, 27, 30 and 31 has been tested at the NCI (Bethesda, USA). The selected compound with remarkable anti-HIV activity (EC50 = 0.09μM) and very high therapeutic index (TI=1177.7) was 9-chloro - 2,3,4 - trihydro - 7 - methyl - 6,6 - dioxopyrimido[1,2-b][1,4,2]benzodithiazine (25).
References:
1. Kuo Ch.L., Assefa H., Kamath S., Brzozowski Z., Sławiński J., Sączewski F., Buolamwini J.K., Neamati N., J. Med. Chem., 2004, 47, 385-399.
2. Brzozowski Z., Sączewski F., Sanchez T., Kuo Ch.L., Gdaniec M., Neamati N., Bioorg. Med. Chem., 2004, 12, 3663-3672.
3. Brzozowski Z., Sączewski F., Neamati N., Bioorg Med. Chem., 2006, in press.
This study was supported by Polish State Committee for Scientific Research (grant no 2 P05 035 27). |