Numerous diarylsulfonylurea derivatives are well known from their anticancer properties. Our extensive study on the synthesis and biological properties of a number of 4- substituted pyridine-3-sulfonamide derivatives [1-5] prompted us to the synthesis and anticancer evaluations of novel series of N-phenyl-N'-(pyridine-3-sulfonyl)urea derivatives.
The synthesis of the target sulfonylurea derivatives were achieved by multi-step reactions starting from 4-chloro-pyridine-3-sulfonamide with N- or S-nucleophiles to produce the corresponding 4-substituted pyridine-3-sulfonamides that were subjected to reactions with suitable phenyl isocyanates to give the expected N-phenyl-N'-(4-substituted pyridine-3-sulfonyl)urea derivatives as shown in scheme below. The structure of this compounds was confirmed by IR, 1H and 13C NMR spectroscopy.
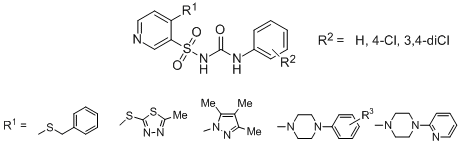
Several compounds were tested in vitro at the National Cancer Institute in Bethesda (USA). This sulfonylureas revealed moderate or reasonable anticancer activity. It could be noticed, that several cancer cell lines exhibit relatively high susceptibility to these sulfonylurea derivatives. Among them, there are cell lines of breast cancer (MDA-MB-468), prostate cancer (PC-3), melanoma (UACC-62 and LOXIMVI) and leukemia (RPMI-8222 and K-562) cell lines. The most active compound N-(4-chlorophenylcarbamoyl)-4-[4-(3,4-dichlorophenyl)piperazin-1-yl]pyridine-3-sulfonamide exhibited significant activity against 7 of human tumor cell lines representing leukemia, colon, melanoma and renal cancer at the low micromolar concentrations, i.e. GI50 ranged from 1.55 to 9.81μM.
References:
1. Brzozowski Z., Sławiński J., et al., J. Heterocyclic Chem., 46, 1396 (2009).
2. Brzozowski Z., Sławiński J., et al., Eur . J. Med. Chem. 45, 3656 (2010).
3. Brzozowski Z., Sławiński J., et al., Eur . J. Med. Chem. 46, 4404 (2011).
4. Brzozowski Z., Sławiński J., et al., Eur. J. Med. Chem. 56, 282 (2012).
5. Sławiński J., Szafrański K., et al., Eur. J. Med. Chem. 69, 701 (2013). |