| Search for content and authors |
Structural and Thermodynamic Properties of III-V and II-VI Ternary Semiconductor Compounds |
| Natalia I. Podolska 1,2,3, Alexander I. Zhmakin 1,3 |
|
1. Ioffe Physico-Technical Institute, RAS, Saint-Petersburg, Russian Federation |
| Abstract |
III-V and II-VI compound semiconductors are basic materials for advanced micro- and nanoelectronic devices such as laser diodes, light emitting diodes, power and microwave transistors etc. To get the best performance from the device, the quality of the epitaxial layers and heterostructures should be steadily improved. To accomplish this aim, the growth methods must rely on the knowledge of the materials' structural and thermodynamic properties. While the methods of the microstructure characterization are being persistently refined, the thermodynamic parameters are extracted from indirect measurements and are not known with sufficient accuracy. The paper presents the result of the valence force field (VFF) simulation of both structural (interatomic distances in the first and second coordination spheres) and thermodynamic properties (the mixing energy, the interaction parameter, the phase diagrams) of the ternary III-V and II-VI compounds (phosphides, antimonides, arsenides, nitrides, tellurides ets.) in the complete composition range. The previously developed statistical model [1,2] is used to compute the phase diagrams. 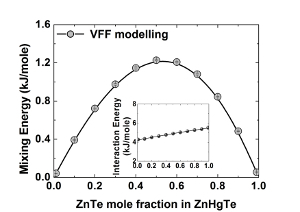 Fig.1: Mixing energy and Interaction energy (inserted graph) as a functions of ZnTe mole fraction in ZnHgTe. Fig.1: Mixing energy and Interaction energy (inserted graph) as a functions of ZnTe mole fraction in ZnHgTe.
The results are compared to both the experimental data and to the theoretical model suggested in refs. [3-5] for the interpretation of the EXAFS data as well as weakness of this the theoretical model.
Acknowledgments This study was supported by the Russian Foundation for Basic Research, the Presidium of the Russian Academy of Sciences, the Presidium of the St. Petersburg Scientific Center of the Russian Academy of Sciences, and the Federal Agency for Science and Innovation. References[1] N.I.Podolska, "Study of thermodynamic properties of multicomponent III-V compounds by valence force field method", PhD Thesis, Ioffe Institute, Saint-Petersburg, Dec. 2011. [2] N.I.Podolska and A.I.Zhmakin, the manuscript will be published soon. [3] B.V.Robouch et al., Eur. Phys. J. B 84, 183-195 (2011). [4] B.V.Robouch et al., J. of Appl. Phys. 104, 073508 (2008). [5] B.V.Robouch et al., J. of Alloys and Compounds 339, 1-17 (2002) |
| Legal notice |
|
| Related papers |
Presentation: Poster at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, Topical Session 4, by Natalia I. PodolskaSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-04-16 01:01 Revised: 2013-04-16 02:12 |