| Search for content and authors |
Growth, structure and magnetic properties of ZnCr2Se4 – single crystals doped by dysprosium. |
| Izabela J. Jendrzejewska 1, Paweł Zajdel 2, Ewa Maciążek 1, Maria Sozańska 3 |
|
1. University of Silesia, Institute of Chemistry (UŚ), Szkolna 9, Katowice 40-006, Poland |
| Abstract |
ZnCr2Se4 is a cubic spinel that exhibits interesting structural and magnetic properties, directly related to the presence of localized 3d electrons of the Cr3+ ions. The presence of magnetic dysprosium in crystal lattice causes essential changes of the physical properties of ZnCr2Se4. Growing of quaternary spinel-type chromium selenide single crystals requires a special version of the chemical transport. The synthesis of the spinel-type selenides was carried out according to the reaction: 4(1–x)ZnSe + 4xDySe + (2+x)CrCl3 = Zn1–xDyxCr2+xSe4 + 3(1–x)ZnCl2 + 3xDyCl3 for x = 0.1÷0.2 The single crystals of ZnCr2Se4 spinel doped with dysprosium were prepared by the chemical vapour transport (CVT) in closed silica tubes using stoichiometric contents of ZnSe, DySe and CrCl3 as the transporting agent. The transporting agent CrCl3 dissociates to CrCl2, CrCl4 and Cl2 above 773K. The transporting reactions, during which CrCl3, CrCl4 and Cl2 are transporting agents were used to calculate the equilibrium coefficients in a heterogeneous system (a gas phase and some solid phases) using the computer program H.S.C. Chemistry 6.1. The ZnSe-DySe-CrCl3 system fulfils conditions for the simultaneous transport of ZnSe and DySe (the same sign of enthalpy and the same value of volatility) and the crystallisation of the Zn1-xDyxCr2+xSe4 - single crystals. The plots for the dependence of the equilibrium constant vs. temperature for the transport reactions 1-9 are shown in Figure 1.
Fig. 1. Dependence of the equilibrium constant Ka on the reaction temperature T for transport with CrCl3 ( reactions 1-3), with CrCl4 (reactions 4-6) and with Cl2 (reactions 7-9). The ampoules containing powdered substrates were placed in a horizontal furnace, where a melting zone temperature of 1173÷1273K and crystallization zone temperature of 1043÷1143K were used. The furnace was cooled during one day after 14 days of heating. Chemical composition of single crystals of Zn1-xDyxCr2Se4-system with different concentration of dysprosium was analyzed using scanning electron microscopy (Figure 2). The single crystals were investigated at room temperature on the Oxford Diffraction Xcalibur diffractometer with Sapphire 3 CCD detector. A black piece of approx dimensions 0.04x0.12x0.12 mm was sliced from the larger octaherdon shaped crystal. The multiscan absorption correction was done using CrysAlis package with estimated transmission in the range T(max) = 1.0, T(min) = 0.152 and data quality factors Rint = 0.060/0.049, Rsig = 0.058/0.008. The structure refinements, taking into account site occupancy factors, indicated that Dy-ions shared octahedral sites with Cr-ions. 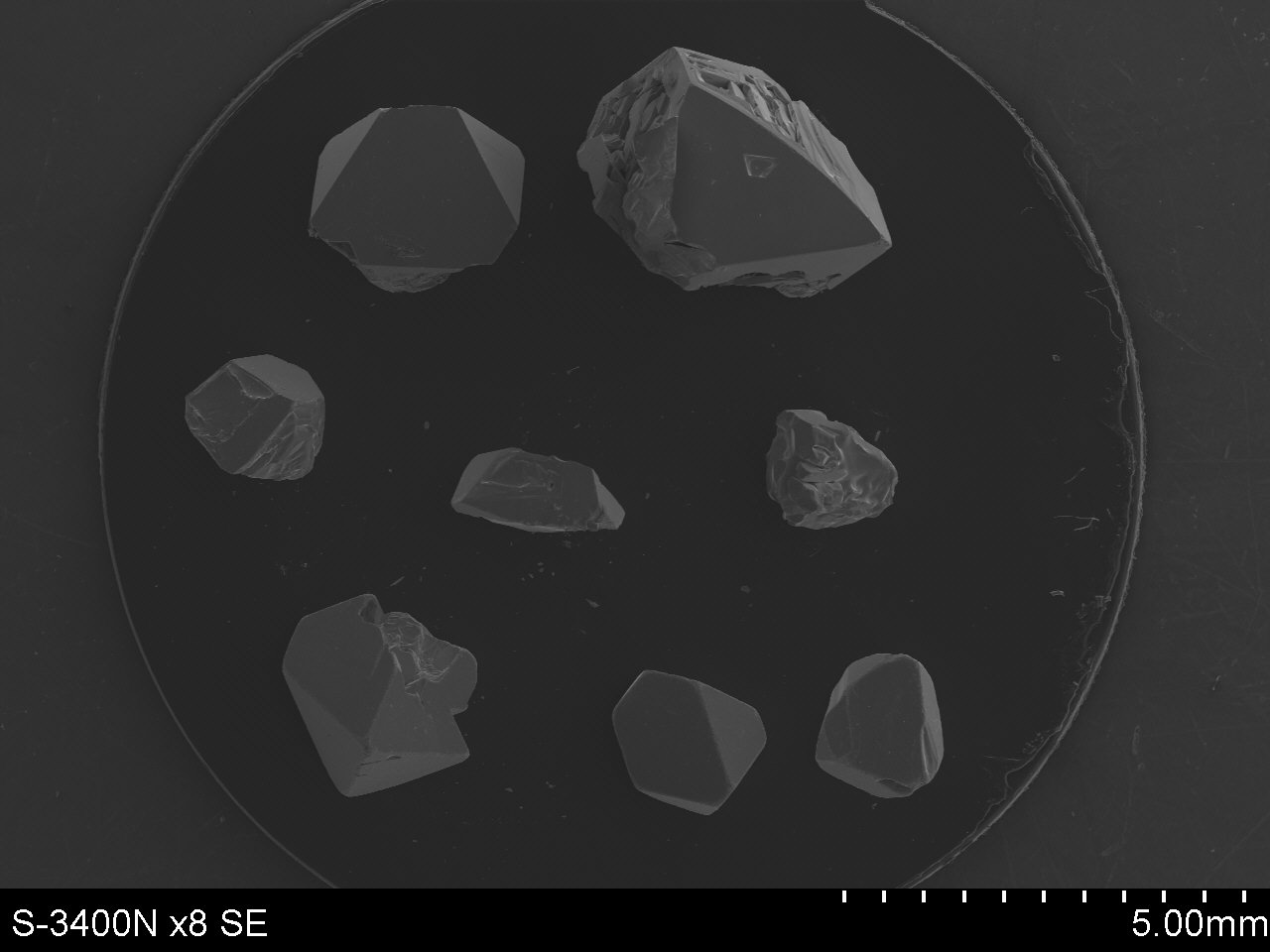
Fig.2. The single crystals of Zn1-xDyxCr2Se4 obtained by CVT-method. The magnetization measurements were carried out over the temperature range 2–300 K using a Quantum Design SQUID – based MPMSXL – 5-type magnetometer with magnetic field of 0.5T and found that saturation moment and transition temperature were affected by the presence of dysprosium. Details of structural and magnetic investigations will be presented. This work is funded from science resources for years 2011-2014 as a research project (project No. N N204 151940). |
| Legal notice |
|
| Related papers |
Presentation: Poster at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, Topical Session 4, by Izabela J. JendrzejewskaSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-03-29 22:41 Revised: 2013-07-18 12:36 |