Spontaneous chiral symmetry breaking during crystallization of sodium chlorate (NaClO3) from aqueous solution has been of great interest. Each molecule of NaClO3 is achiral, but they forms chiral cubic crystals (space group P213). Crystallization from a static solution by evaporation yields statistically equal numbers of L- and D-crystals because of equal thermodynamic stability of the two enentiomorphs. Nevertheless, if the solution is continuously stirred during crystallization, resulting crystals are almost all L or all D in each crystallization experiment [1]. This significant imbalance in chirality is referred to as chiral symmetry breaking. Mechanism of this spontaneous symmetry breaking is not fully understood yet.
Despite of many interpretations and implications of the symmetry breaking during early stage of crystallization, there have been few direct observations in the early stage of crystallization from the viewpoint of the chirality. Recently, we performed an in-situ observation of nucleation induced by droplet-evaporation with a polarized-light microscope, and we for the first time found that an unstable unknown crystalline phase nucleates prior to appearance of the chiral crystal [2]. However, owing to lack of information of the unknown phase, its role in the determination of chirality is unclear.
In this study, chirality and stability of the unknown phase were evaluated by a cryogenic single-crystal X-ray structure-analysis and solubility measurements respectively. Moreover, we also performed an in-situ observation of phase transition from the unknown phase into the chiral phase by using a polarized-light microscope. The microscope is capable to identify handedness of the chiral crystal.
As the result of the evaluation of the unknown phase, (1) Lattice constant, crystal system and space group were determined as follows: a = 8.42 (Å), b = 5.26 (Å), c = 6.70 (Å), β = 109.71(°), monoclinic, and P21/a, respectively. The space group P21/a implies that the unknown phase is achiral. (2) Solubility was roughly determined to be 1.6 times higher than that of the chiral cubic phase in the range from 10 °C to 23 °C, meaning that the unknown phase is metastable phase. For (1), (2) and the fact that the metastable phase transforms into the chiral cubic phase, we concluded that the unknown phase plays the role of precursor of chiral crystals.
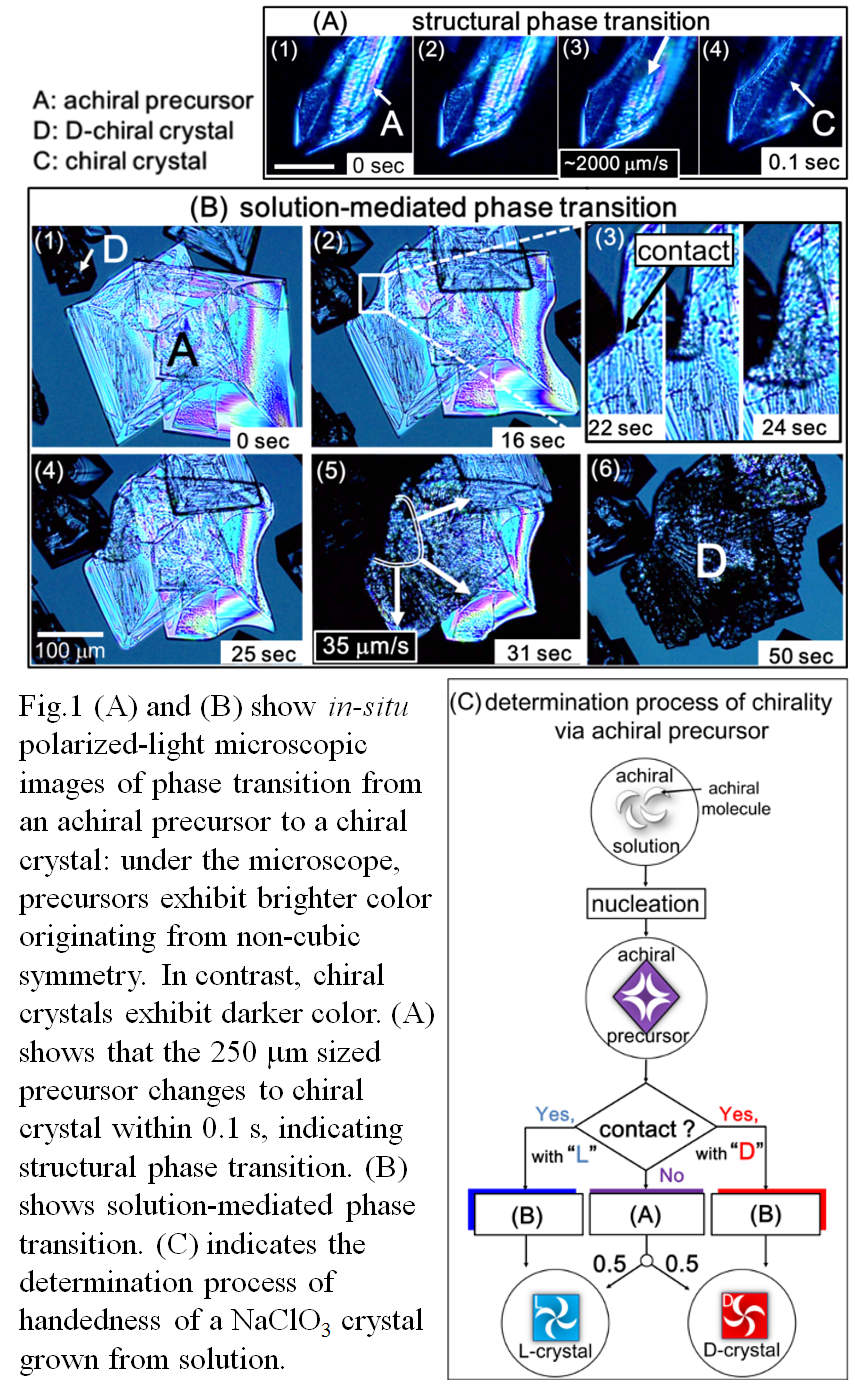
In addition, the in-situ observation showed that the phase transition is classified into two types according to transition rate. Fig. 1 (A) shows that a bright precursor rapidly changes into a dark chiral crystal with transition rate of ~2000 μm/s. In contrast, Fig. 1 (B) shows slow phase transition with transition rate of ~35 μm/s [Fig. 1 (B) (5)]. This large difference of the transition rate is considered to be due to difference of mechanism. Namely, the rapid transition should be structural phase transition governed by intracrystalline displacement of atoms, and the slow transition should be solution-mediated phase transition governed by dissolution/precipitation. Peculiarly, as shown in Fig. 1 (B) (3), the solution-mediated phase transition is induced by contact of a precursor with a chiral crystal. In this case, the precursor transforms into a chiral crystal with the same handedness as the contacted chiral crystal [Fig. 1 (B) (6)].
We concluded that the precursor transforms into chiral crystal by either solution-mediated phase transition or structural phase transition. If the precursor contacts with a chiral crystal, it transforms by the solution-mediated phase transition. Whereas handedness is determined randomly in the case of the structural phase transformation, handedness results in the same as that of contacted crystal in the case of solution-mediated phase transformation [Fig. 1 (C)].Beside the direct nucleation of the chiral crystal, the structural phase transition of the achiral precursor has a potential to bear the origin of handedness. Preferential choice of handedness seen in the solution-mediated phase transition may lead to chiral symmetry breaking.
References
[1] D.K. Kondepudi, R.J. Kaufman, N. Singh, Science 1990, 250, 975-976. [2] H. Niinomi, K. Tsukamoto, M. Uwaha, H. Miura, Japan Geoscience Union Meeting 2010, MIS012-06. |