| Search for content and authors |
Magnetic and optical properties of Li0.28Na1.72Ge4O9:Cr, Mn (0.1mol%) single crystals |
| Slawomir M. Kaczmarek 1, Taiju Tsuboi 3, Yoshio Nakai 3, Marek Berkowski 2, Grzegorz Leniec 1, Anna Leniec 1 |
|
1. West Pomeranian University of Technology, Szczecin (ZUT), Szczecin 70-310, Poland |
| Abstract |
Li0.28Na1.72Ge4O9:Cr (0.1mol.%) and Li0.28Na1.72Ge4O9:Cr, Mn (0.1mol%, 0.1mol.%) (LNG) single crystals have been prepared by Czochralski method. They were investigated by electron paramagnetic resonance (EPR) and photoluminescence (PL) in a temperature range of 3-300 K. EPR signal from Cr3+ ions shows at least two magnetically nonequivalent centers and some features characteristic of dissimilar ion pairs transitions (chromium and sodium), that are found from roadmap at high magnetic fields (over 4000 Gs) in limited range of angles. Manganese ion enters as divalent ion and reveals different locations. The area in roadmap where dissimilar pairs transitions are located is split in a case of Mn codoped crystal. 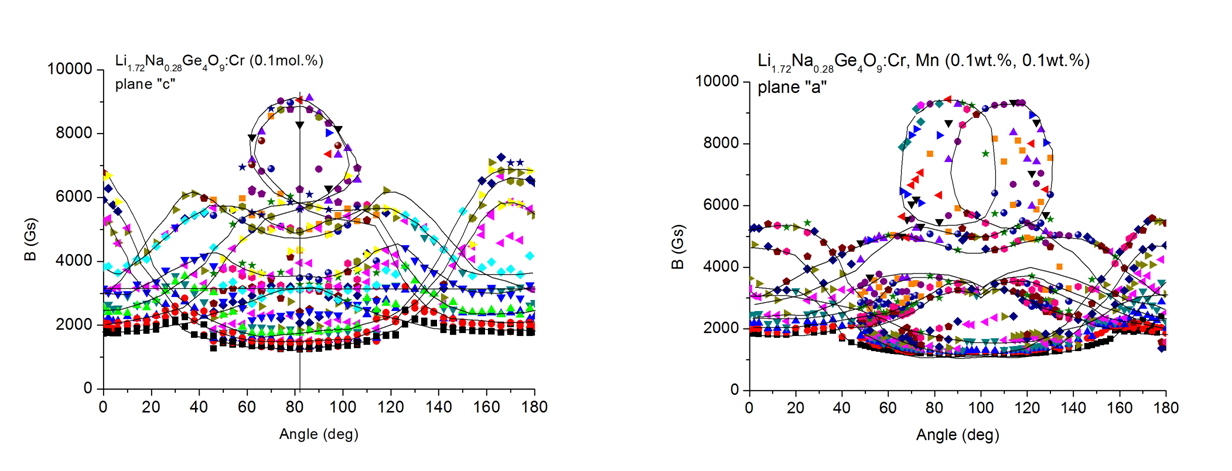
PL spectra show two bands centered at about 425 nm and 695 nm, which are attributable to chromium ions in disordered matrices. Co-doping with Mn2+ leads to energy transfer to chromium ions. The maximum of PL for LNG:Cr, Mn is about 20 times higher than that for chromium only doped crystal. 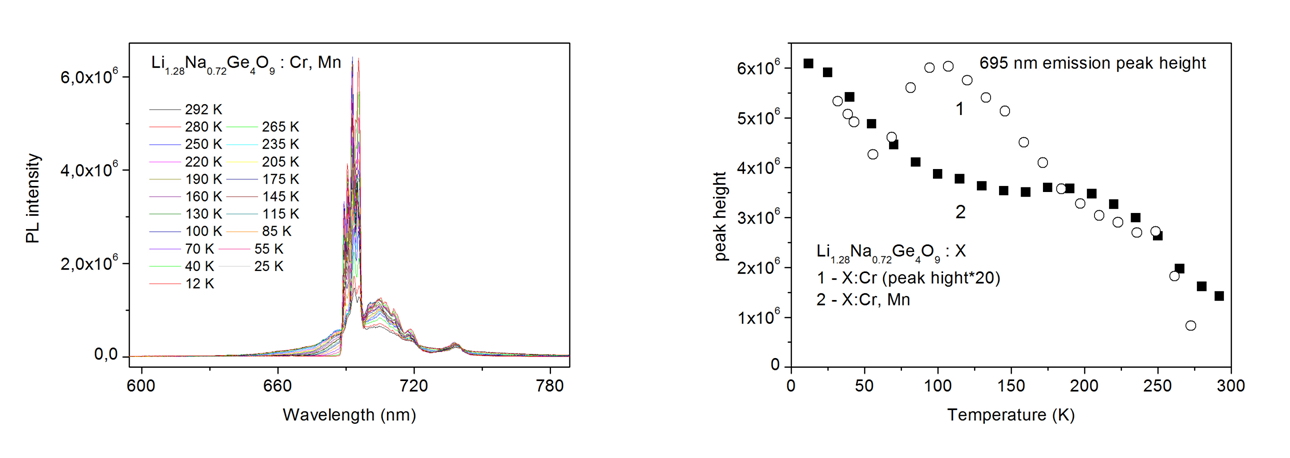 |
| Legal notice |
|
| Related papers |
Presentation: Oral at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, Topical Session 6, by Slawomir M. KaczmarekSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-03-23 16:17 Revised: 2013-07-18 13:35 |