| Search for content and authors |
Complete deracemisation of proteinogenic amino acids using Viedma ripening |
| Laura Spix , Hugo Meekes , Willem J. Van Enckevoort , Elias Vlieg |
|
Radboud University Nijmegen, Heyendaalseweg 135, Nijmegen 6525AJ, Netherlands |
| Abstract |
| Viedma ripening is a deracemisation method in which crystals in a saturated solution are vigorously ground and the chiral molecules are simultaneously racemised in the solution.[1] This leads to an enantiomerically pure solid state if the chiral molecules crystallize as a conglomerate. A conglomerate is equal to a mechanical mixture of pure (R) and (S) crystals. Unfortunately 90 % of the organic crystals form a racemic compound in which molecules of both handedness are present in the unit cell.[2] Hence they cannot be deracemised using Viedma ripening. We will present here two methods that allow a complete deracemisation on intrinsically racemic compound forming proteinogenic amino acids.
In the first case we make use of Ostwald’s rule of stages (thermodynamically unstable phases appear first followed by recrystallisation to the thermodynamically stable phase). Glutamic acid forms a stable racemic compound.[3] Under well chosen experimental conditions a metastable conglomerate is obtained and its stability is sufficient to enable a complete deracemisation.[4] 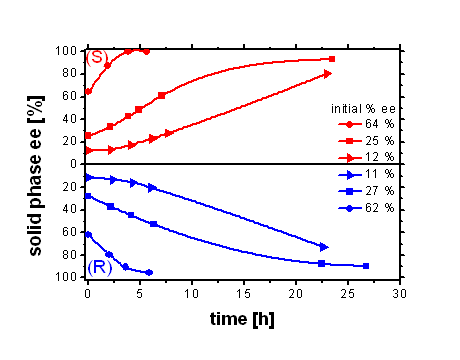
Figure 1: Attrition-enhanced evolution of solid-phase ee for Glutamic acid at 90 °C (upper) and Alanine 4-chlorobenzenesulfonate (Ala-CBS) at 70 °C. (lower) starting from initial values of the ee as shown. In the second case a conversion into a salt is used. Alanine crystallizes in a 3D network structure as a stable racemic compound. Transformation into a 4-chlorobenzenesulfonate leads to a conglomerate with a layered structure.[5] The sulfonate conglomerate enables a complete deracemisation. The enantiomerically pure final state has in both cases (Glu and Ala-CBS) a limited life time when left in the slurry. Glutamic acid transforms after a certain time into the most stable crystal structure which is a racemic compound and returns to a racemic state under deracemisation conditions. The alanine salt shows a different behavior. Sometimes the enantiomeric excess of the solid phase decreases after having reached 100 %. The epitaxial growth of the crystals could be the reason for this effect. (see picture 1)
picture 1: Etching experiment of an Ala-CBS crystal in a saturated solution of L-Ala-CBS (under the polarisation microscope) [1] a)C. Viedma, Phys. Rev. Lett. 2005, 94, 065504; b)W. L. Noorduin, T. Izumi, A. Millemaggi, M. Leeman, H. Meekes, W. J. P. Van Enckevort, R. M. Kellogg, B. Kaptein, E. Vlieg, D. G. Blackmond, J. Am. Chem. Soc. 2008, 130, 1158; c)S. Yamada, C. Hongo, R. Yoshioka, I. Chibata, J. Org. Chem. 1983, 48, 843. [2] A. C. Jean Jacques, Samuel H. Wilen, Enantiomers, Racemates, and Resolutions, Krieger, Malabar, Florida, 1994. [3] J. D. Dunitz, W. B. Schweizer, Acta Cryst. Sec. C 1995, 51, 1377. [4] L. Spix, H. Meekes, R. H. Blaauw, W. J. P. van Enckevort, E. Vlieg, Cryst. Growth Des. 2012, 12, 5796. [5] H. Kimoto, K. Saigo, Y. Ohashi, M. Hasegawa, Bull. Chem. Soc. Jpn. 1989, 62, 2189. |
| Legal notice |
|
| Related papers |
Presentation: Oral at 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17, General Session 3, by Laura SpixSee On-line Journal of 17th International Conference on Crystal Growth and Epitaxy - ICCGE-17 Submitted: 2013-03-14 15:04 Revised: 2013-07-17 10:19 |