| Search for content and authors |
Physicochemical characterization of sunitinib and its impurities |
| Katarzyna Sidoryk 1, Krzysztof Bańkowski 1, Marek Kubiszewski 1, Marta Łaszcz 1, Magdalena Bodziachowska-Panfil 1, Magdalena Kossykowska 1, Maura Maliska 2, Krzysztof Woźniak 2 |
|
1. Pharmaceutical Research Institute, Rydygiera 8, Warsaw 01-793, Poland |
| Abstract |
Sunitinib is an oral small-molecule tyrosine kinase inhibitor (TKI) that targets and blocks the signaling pathways of multiple selected receptor tyrosine kinases (RTKs). Sunitinib was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GITS) on January 26, 2006. During the investigation on the synthesis of pharmaceutically pure sunitinib L-malate based on the procedure described in EP 1255752B1 (Sugen Inc. and Pharmacia & Upjohn Co.) the physicochemical studies of sunitinib as well as its potential degradation products and process-related impurities were performed. The structure elucidation of the compounds was accomplished by means of the following techniques: NMR, IR and MS. Furthermore, X-ray single-cristal studies of sunitinib unambiguously proved the structure. A selective HPLC method for the determination of chemical purity of sunitinib was applied. 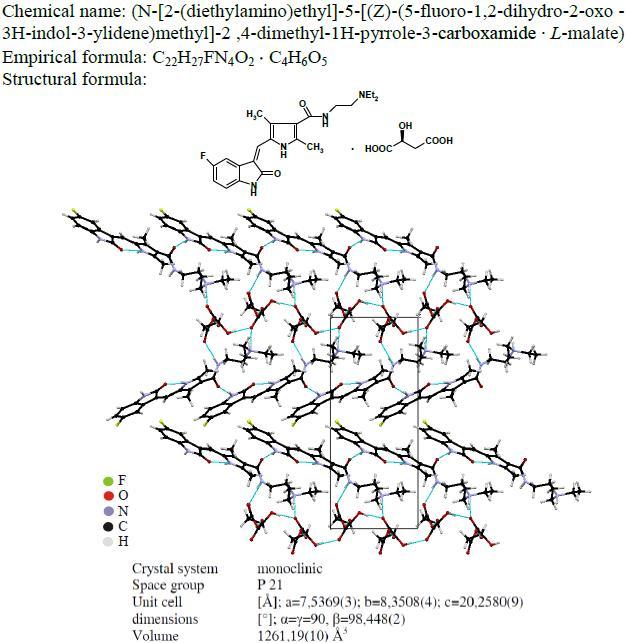
Research Project is supported by European Union, Project no UDA-POIG.01.03.01-14-069/08-00, 26.02.2009„Innovative technologies of oncological medicines of special therapeutic and social importance”. |
| Legal notice |
|
| Related papers |
Presentation: Poster at VIII Multidyscyplinarna Konferencja Nauki o Leku, by Krzysztof BańkowskiSee On-line Journal of VIII Multidyscyplinarna Konferencja Nauki o Leku Submitted: 2012-03-14 10:56 Revised: 2012-03-20 12:34 |